| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 6, Number 4, August 2016, pages 72-80
The Indication for Long-Term Oral Antiplatelet Therapy After Endovascular Embolization of Unruptured Intracranial Aneurysms
Kei Haradaa, d, Kohsuke Kakumotoa, b, Shogo Oshikataa, Kenji Udab, c, Masahito Kajiharaa, Naoki Higoa, Yukuhiro Sankodab, Yuto Hatanoc, Shunsuke Taniguchia
aDepartment of Neurosurgery, Fukuoka Wajiro Hospital, 2-2-75, Wajirogaoka, Higashi-Ku, Fukuoka-city, Fukuoka 811-0213, Japan
bDepartment of Neurosurgery, Fukuoka Shinmizumaki Hospital, Tateyashiki, 1-2-1, Mizumaki-chou, Onga-gun, Fukuoka 807-0051, Japan
cDepartment of Neurosurgery, Shin-Yukuhashi Hospital, Doujouji 1411, Yukuhashi-City, Fukuoka 824-0026, Japan
dCorresponding Author: Kei Harada, Department of Neurosurgery, Fukuoka Wajiro Hospital, 2-2-75, Wajirogaoka, Higashi-Ku, Fukuoka-city, Fukuoka 811-0213, Japan
Manuscript accepted for publication August 24, 2016
Short title: Antiplatelet Therapy of Cerebral Aneurysms
doi: http://dx.doi.org/10.14740/jnr390w
| Abstract | ▴Top |
Background: Antiplatelet therapy (APT) is indispensable to prevent ischemic complications of unruptured intracranial aneurysms (UICAs); however, long-term APT can cause hemorrhagic complications. We evaluated the rates of use and factors for long-term APT in patients who underwent endovascular embolization of UICA.
Methods: A total of 175 UICAs in 169 patients were studied. Dual APT was initiated, pre-operatively. Embolization of the UICAs was achieved with four different endovascular methods: single/double catheter (41% of the cases), balloon-assisted (50%), stent-assisted (9%), and internal trapping (2%). Single APT was utilized for 1 - 3 months in patients who were not treated with a stent. In patients who were treated with the stent-assisted method, dual APT was continued for 3 - 6 months, with transition to single APT.
Results: The rates of pre-operative APT were 13%, 87%, and 0.6% in the single, dual, and triple APT therapy groups, respectively. APT was necessary for over 1 year in 18% of patients; 11% of the patients needed long-term PT because of aneurysm-specific factors (20 cases: stent placement, 16; stenosis of the branch artery of the aneurysm, one; coil protrusion to the parent artery, two; coil migration, one), and the indication was patient-specific for 7% (nine cases: coronary artery disease, five; cerebral artery disease, four). Delayed ischemic events occurred in 1.1% (two cases); one cerebral infarction was associated with stent-assisted embolization and one occurred due to stenosis of the branching artery.
Conclusions: The most common indication for long-term APT was stent placement. Stenosis of the branch artery of the aneurysm, coil protrusion into the parent artery, and coil migration were the other reasons for long-term APT. The use of stents should be limited to the management of wide-neck or large aneurysms to prevent delayed ischemic and hemorrhagic complications.
Keywords: Unruptured intracranial aneurysm; Endovascular embolization; Antiplatelet therapy; Stent-assisted embolization
| Introduction | ▴Top |
Endovascular embolization is now a standard therapeutic option for intracranial aneurysms. Recently, stent-assisted embolization for cerebral aneurysms (SAC) has become popular; however, antiplatelet therapy (APT) is needed to prevent delayed ischemic strokes after SAC. After SAC, the standard course of treatment includes 3 - 9 months of dual APT (DAPT) with aspirin and clopidogrel, followed by single APT (SAPT), with discontinuation of APT. It has not been determined whether APT is needed after embolization of intracranial aneurysms that involve coil protrusion from the aneurysm to the parent artery, or coil migration into the distal cerebral arteries. In this study, the rates of use and indications for APT after endovascular embolization for unruptured intracranial aneurysm (UICA) were retrospectively studied. In addition, we report on the complications of APT that occurred after coil embolization for UICA.
| Materials and Methods | ▴Top |
We retrospectively analyzed the clinical and radiologic data of 226 patients with UICA who underwent endovascular embolization between January 2010 and June 2013 in our associated hospitals. These cases were considered suitable for embolization after evaluation by neurosurgeons and neurointerventionalists, or because the patient refused clipping. Informed consent of the endovascular treatment was obtained in all patients. This study included patients who underwent endovascular embolization and were then followed up for over 1 year. Patients with UICAs associated with ruptured aneurysms, those who underwent re-treatment, and those who did not present for follow-up for over 1 year, were excluded. A total of 175 UICAs in 169 patients were included. The sample included 71% of females, and the mean patient age was 60.2 years (range, 27 - 84 years). The mean maximum aneurysm diameter was 7.3 mm (range, 2.0 - 22 mm). Aneurysmal locations were the internal carotid artery (ICA) in 64%, anterior communicating artery (Acom) in 6%, anterior cerebral artery (ACA) in 3%, middle cerebral artery (MCA) in 5%, vertebral artery in 5%, basilar artery in 16%, and posterior cerebral artery in 1%. DAPT (aspirin, 100 mg; clopidogrel, 75 mg daily) was administered for more than 4 days before endovascular embolization. A total of 98% of aneurysms were treated with embolization, and 2% were treated with internal trapping. Embolization of the aneurysm was performed using the simple/double catheter, balloon-assisted, and stent-assisted technique in 41%, 50%, and 9% of aneurysms, respectively. Systemic heparinization was performed to achieve a value of about 250 s of activated coagulation time.
Postoperatively, anti-coagulation therapy (heparin or argatroban) was prescribed, if necessary. In cases where SAC was not performed, SAPT (aspirin 100 mg daily or clopidogrel 75 mg daily) was prescribed for 1 - 3 months, and then APT was discontinued. In cases of SAC, DAPT was prescribed for 3 - 6 months and then SAPT was continued over 1 year (aspirin, clopidogrel, or cilostazol).
| Results | ▴Top |
Technical success was achieved in 98.9% of cases. RS1, 2, and 3 were obtained in 55%, 23%, and 23% of cases, respectively. Postoperatively, ischemic complications with transient neurological symptoms occurred in 2.3% (four cases), and permanent neurological symptoms were developed in 2.3% (four cases: narrowing of visual field, two; hemi-sensory disturbance, one; trunk ataxia, one).
In 93% of patients, DSA or MRA was performed 6 months or more after the procedure. Recurrence was performed identified in 8.0% (14 cases) with a mean follow-up period of 19 months, and retreatment with endovascular embolization was performed in 2.9% (five cases).
Delayed ischemic events occurred in 1.1% (two cases) (Figs. 1 and 2). One of them was a large Acom large aneurysm treated with SAC (Fig. 1). The patient received DAPT with 100 mg of aspirin and 75 mg of clopidogrel. Postoperatively, 10,000 units per day of heparin were continued. The day after SAC, the patient experienced transient left hemiparesis and diffusion-weighted imaging (DWI) revealed multiple spotty new infarctions. In this case, malposition of the stent was thought to be the cause of a delayed embolism. Triple APT was continued for 2 months with 200 mg of cilostazol, 100 mg of aspirin, and 75 mg of clopidogrel. Neurological deficit was completely recovered. DPT of aspirin and clopidogrel was continued for additional 4 months, and SAPT with aspirin was continued. Other case was patient with basilar superior cerebellar artery (SCA) aneurysm (Fig. 2). The patient received APT of oral 100 mg of aspirin and 75 mg of clopidogrel. The clopidogrel was then discontinued postoperatively. Twelve days after embolization with the simple technique, the patient suddenly developed trunk ataxia and right hemi-ataxia. DWI revealed a new right cerebellar new infarction with an occluded SCA. The patient was diagnosed with thrombus formation after coil embolization. The patient received injection for 7 days and DAPT with aspirin and clopidogrel for 6 months, and was maintained on SAPT with aspirin.
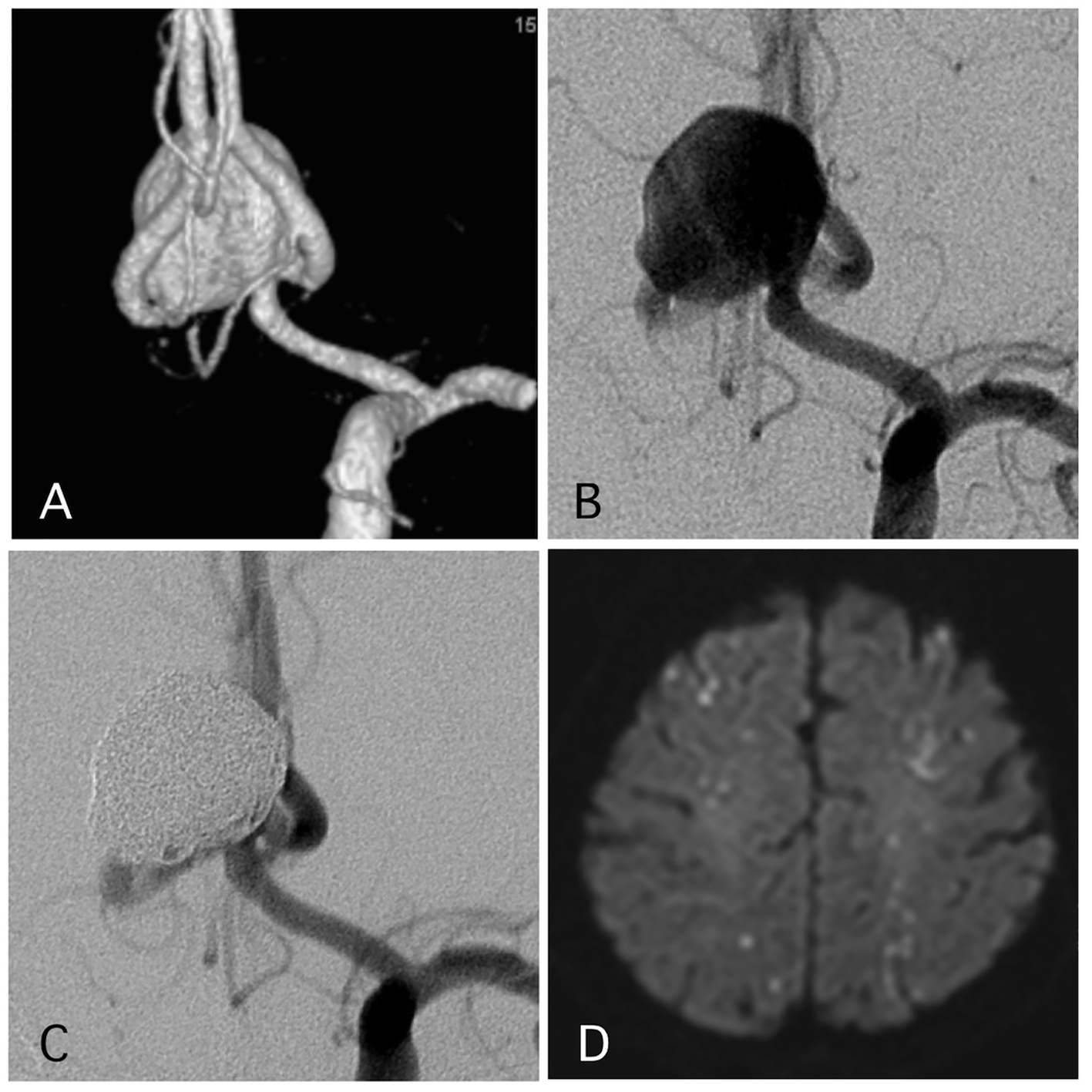 Click for large image | Figure 1. A representative case of a post-procedural ischemic stroke. (A) Pre-operative angiography shows a wide-neck aneurysm of the anterior communicating artery with a maximum diameter of 17 mm. (B) Pre-operative angiography. (C) Postoperative angiography reveals complete occlusion of the aneurysm with assisted stent. (D) The patient experienced transient left hemiparesis 1 day after the procedure. The antiplatelet and anti-coagulation therapies were intensified. |
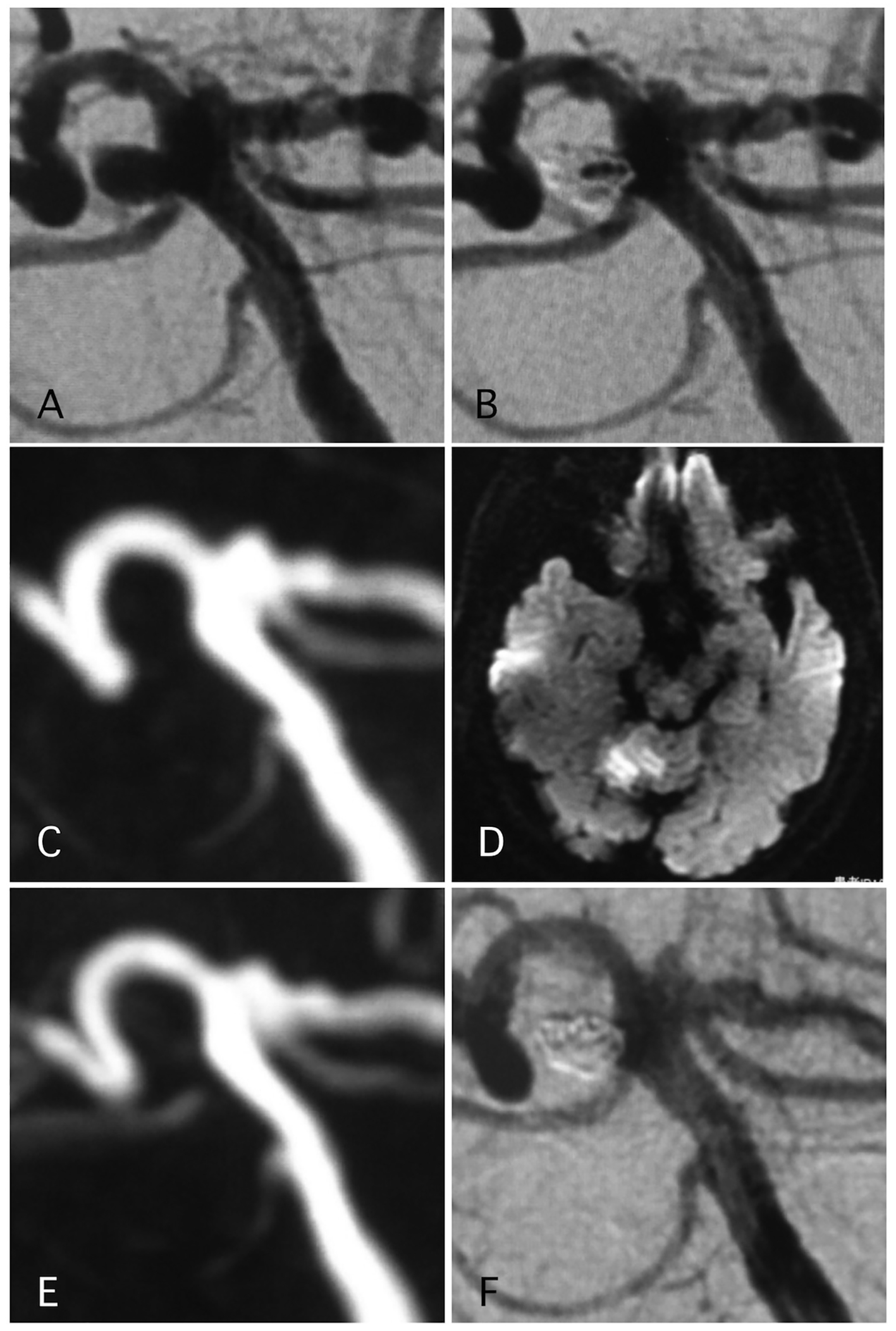 Click for large image | Figure 2. A representative case of post-procedural ischemic stroke. (A) Pre-operative angiography shows basilar-right superior cerebellar artery (SCA) aneurysm. (B) Postoperative angiography shows dome filling of the aneurysm. (C, D) MRI-DWI and MRA 12 days after the procedure, due to sudden trunk ataxia. Left cerebellar infarction occurred due to thrombosis of right SCA. The arrow shows stenosis of the SCA. Antiplatelet and anti-coagulation therapies were adjusted. (E) MRA 25 days after the procedure demonstrated recanalization of the SCA with weak signal of origin of right SCA. (F) Angiography 6 months after the procedure shows complete occlusion of the aneurysm and complete recanalization of right SCA. |
Pre-operative APT
The durations of the preoperative and postoperative APT are shown in Figure 3. Preoperatively, the rates of SAPT, DAPT, and triple APT were 12.0%, 87.4%, and 0.6%, respectively. All of the patients receiving SAPT were on aspirin. In the patients receiving DAPT, 98.9% were receiving the combination of aspirin and clopidogrel, and 1.1% were receiving aspirin and cilostazol 1.1%. Triple APT was combination of aspirin, clopidogrel, and cilostazol. In nine cases, APT had been initiated for indications other than UICA: ischemic heart disease or coronary stent placement in five cases, and cerebral infarction or cerebra artery stenosis in four cases. There were no major hemorrhagic complications such as cerebral hemorrhages or gastric bleeding requiring blood transfusions.
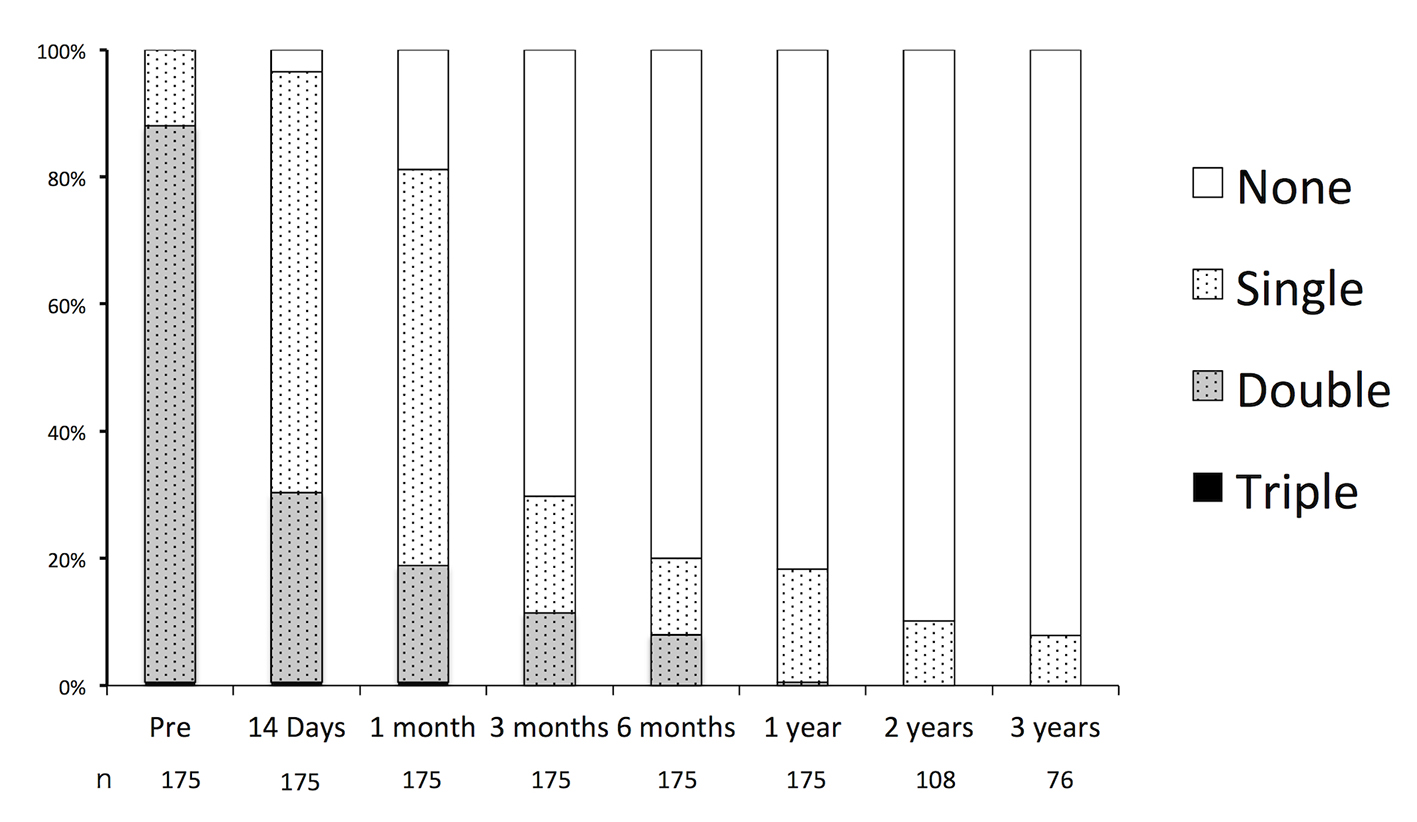 Click for large image | Figure 3. Durations of oral antiplatelet therapy before and after endovascular embolization of unruptured intracranial aneurysms. |
Postoperative anti-coagulation therapy and APT
Postoperatively, 53% of patients received anti-coagulation therapy (heparin or argatroban) for 24 - 48 h. All patients who underwent SAC received postoperative anti-coagulation therapy.
Postoperatively, APT was continued in 96.5%, 81.1%, 29.7%, 20.0%, and 18.3% of patients, and it was maintained for 14 days, 1 month, 3 months, 6 months, and 12 months, respectively. Of the patients who received over 12 months of APT, the indications were aneurysmal factors in 62% of cases and patient-specific factors in 38%. Factors and rates of oral APT after endovascular embolization of UICA over 1 year are shown in Table 1.
 Click to view | Table 1. Factors and Rates of Oral Antiplatelet Therapy After Endovascular Embolization of Unruptured Intracranial Aneurysms Over 1 Year |
APT after aneurysmal internal trapping
Internal trapping was performed in 3.4% (four cases). The locations of the aneurysms were ICA in one, vertebral artery in one, and posterior cerebral artery in two. In two of the cases, DAPT and SAPT were discontinued after 3 and 6 months, respectively. In one case, DAPT was discontinued the day after the procedure, and SAPT was discontinued after 1 month. In one case, DAPT was discontinued after 1 month, and SAPT was continued for over 4 years.
APT after coil protrusion to the parent artery
Coil protrusion into the parent artery occurred in 4.0% of cases. In five of the cases, the coil protrusion of the coil measured 2 mm to 1 cm. In two of those three cases, DAPT and SAPT were continued for 1 and 3 months, respectively. In one of those three cases, APT was continued because the case involved other complications in addition to the distal migration (Fig. 4). In two of the cases, coil loop protrusion into the parent artery occurred (Figs. 5 and 6). In one case, DAPT and SAPT were continued for 1 and 3 months, respectively. In the other case, the coil was maintained in the aneurysm during the procedure; however, a skull X-ray the day after the procedure revealed coil loop protrusion into the parent artery with no associated symptoms (Fig. 5). In this case, DAPT was continued for 3 months and SAPT was continued over 4 years. No delayed ischemic events occurred in any of the cases involving coil protrusion into the parent arteries.
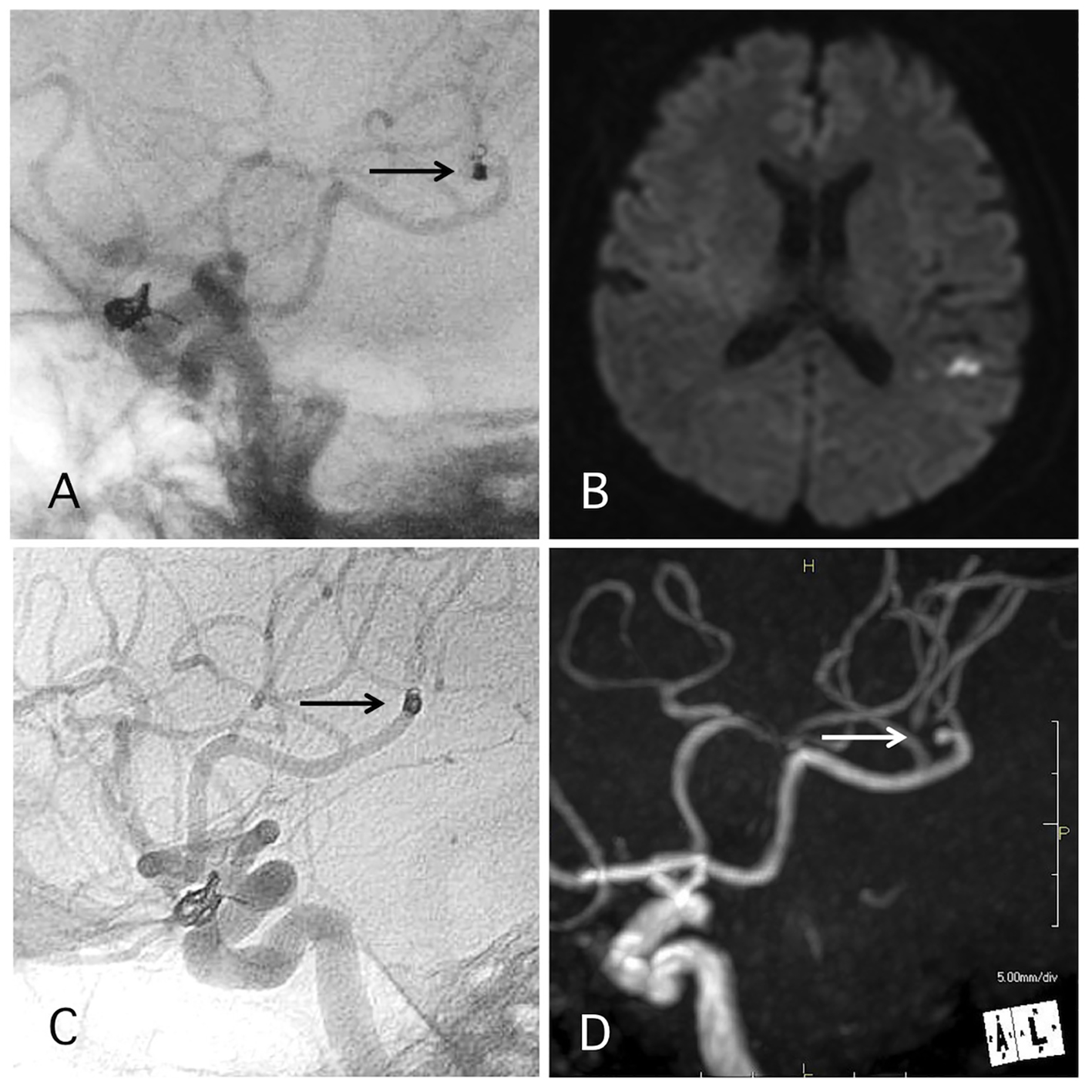 Click for large image | Figure 4. A representative case of post-procedural coil migration. (A) Postoperative angiography shows complete occlusion of the internal carotid artery-ophthalmic artery aneurysm with slight coil protrusion and coil migration to the middle cerebral artery (MCA). Antiplatelet therapies were continued. (B) MRI-DWI 1 day after the procedure shows cerebral infarction. (C) Angiography 1 year after the procedure shows antegrade flow of MCA through the migrated coil. (D) MRA 4 years after the procedure shows signal defect (arrow) due to the migrated coil and distal cerebral blood flow (double arrow). |
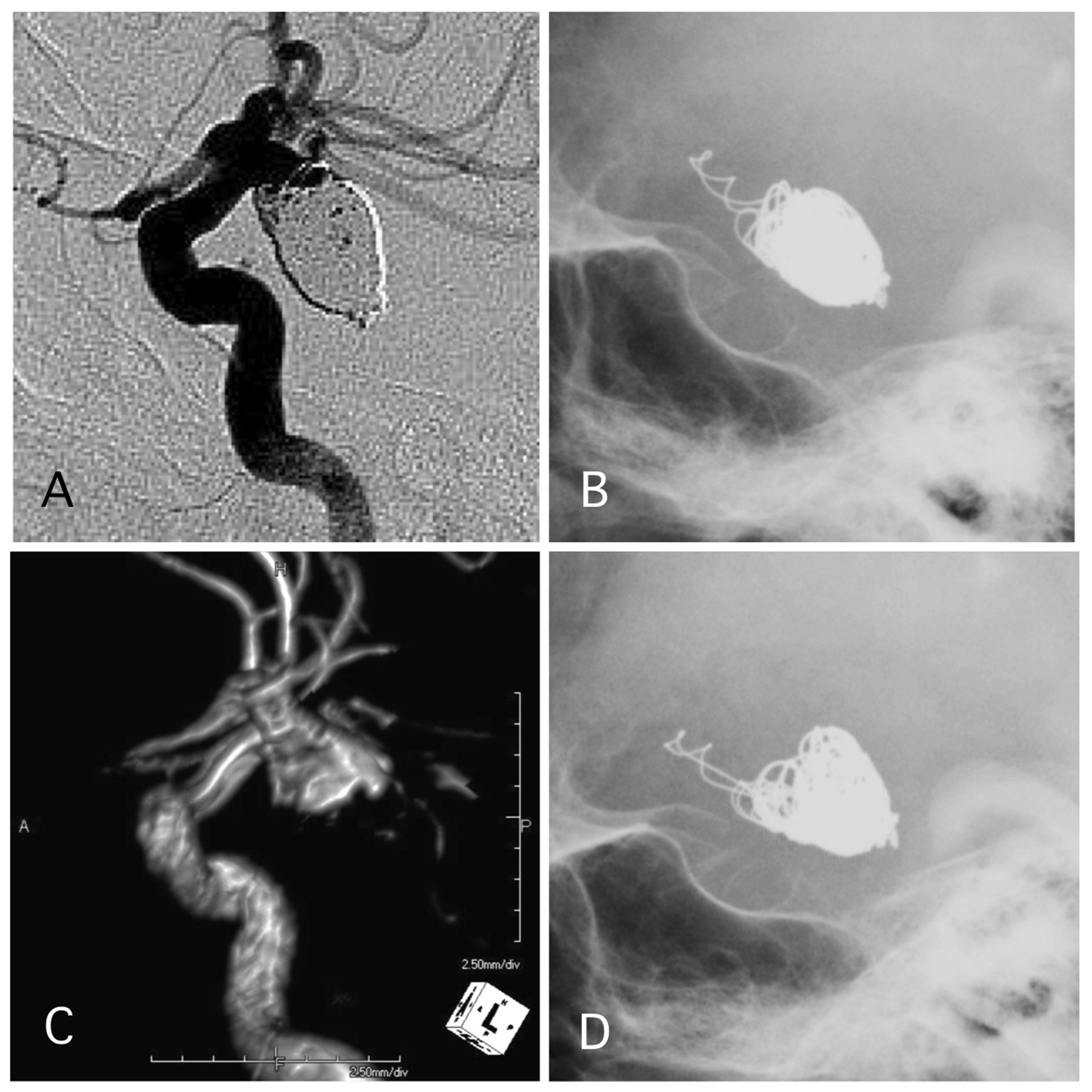 Click for large image | Figure 5. A representative case of post-procedural coil protrusion. (A) Postoperative angiography shows neck remnant of the internal carotid artery-posterior communicating aneurysm with no coil protrusion. (B) Skull X-ray 1 day after the procedure shows coil protrusion. Antiplatelet therapy was continued. (C) MRA 4 years after the procedure shows neck remnant of the aneurysm and no stenotic change in the internal carotid artery. (D) Skull X-ray 4 years after the procedure shows coil protrusion. |
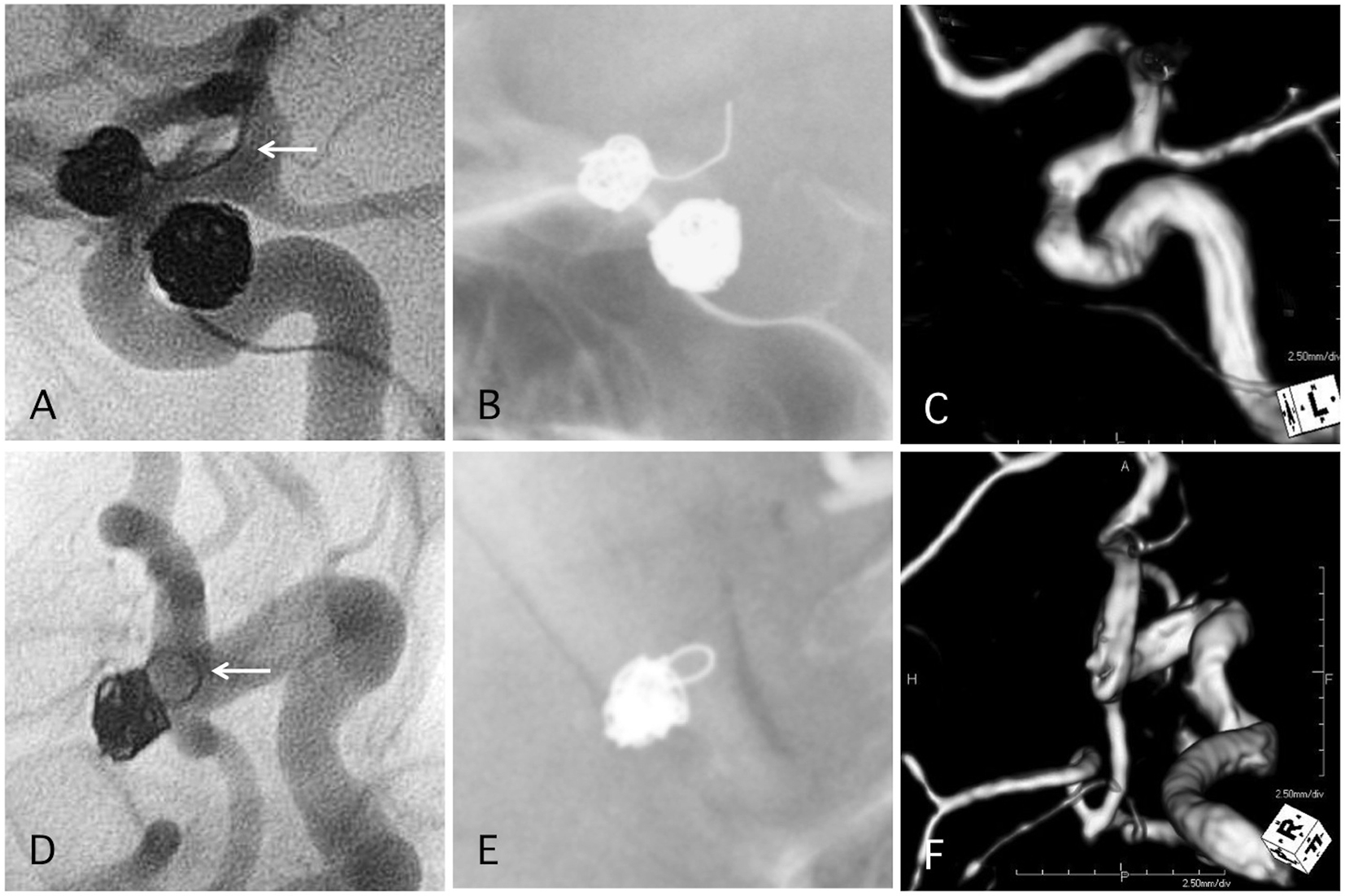 Click for large image | Figure 6. Two representative cases of post-procedural coil protrusion. (A) Postoperative angiography shows complete occlusion of the internal carotid artery aneurysm with one loop coil protrusion (arrow). Antiplatelet therapy is discontinued after 3months. (B) Skull X-ray 4 years after the procedure. (C) MRA 4 years after the procedure shows complete occlusion of the aneurysm and no stenotic change in the internal carotid artery. (D) Postoperative angiography shows complete occlusion of the internal carotid artery-posterior communicating aneurysm with one loop coil protrusion. Antiplatelet therapy is discontinued after 3months. (E) Skull X-ray 4 years after the procedure. (F) MRA 4 years after the procedure shows complete occlusion of the aneurysm and no stenotic change in the internal carotid artery. |
APT after coil migration to the normal cerebral artery
In 0.6% of the cases, coil migration into the normal cerebral artery occurred. In this case, 1.5 mm to 1 cm of the end of the finishing coil migrated into the distal MCA during embolization of the aneurysm located in the ICA (Fig. 4). Retrieval of the coil by an endovascular or surgical approach would have been difficult because it involved the distal portion; moreover, no neurological deficits occurred, anti-coagulation therapy using heparin postoperatively for 7 days, and the patient was maintained on DAPT for 1 year. MCA flow at the portion of migrated coil was evaluated by angiography 1 year and by MRA 4 years after the procedure. Then, SAPT with 75 mg of clopidogrel daily was continued over 5 years, and the patient experienced no ischemic events.
| Discussion | ▴Top |
Long-term APT brings the risk of bleeding, and DAPT is associated with a significantly increased risk of bleeding events for secondary stroke prevention [1].
APT after embolization of UICA reduced thromboembolic events [2]. In cases involving successful coil embolization of saccular aneurysms that were treated without a stent and had no protrusions of the coils to the parent artery, ischemic complications rarely occurred during the first 1 - 3 months on SAPT after the procedure. On the other hand, treatment with SAC enabled embolization of wide neck and large aneurysms, and delayed ischemic events after SAC are unresolved.
APT and delayed ischemic event after SAC
In patients who undergo SAC for UICA, pre-operative treatment with DAPT (aspirin, 100 mg; clopidogrel, 75 mg) for more than 3 days before the treatment is generally performed [2-9]. After SAC, DAPT with aspirin and clopidogrel was continued for 6 weeks to 9 months, and delayed ischemic strokes occurred in 0.6-16% [3-14]. Moreover, ischemic events were more likely to occur after the transition from DAPT to SAPT [5, 9, 10, 13, 14]. Hwang et al reported rates of delayed ischemic events of 3.5% within 2 months following the switch from DAPT to SAPT [5]. Giant aneurysms, fusiform AN, incomplete occlusions, Y-stents, waffle-cone stents, stent malpositioning, Enterprise stent, and coil loop prolapse are all reported to increase the risk of delayed ischemic events after SAC [5, 7, 8, 10, 12]. Delayed in-stent restenosis is also reported to be a late complication in 2.6-14% of cases [3, 7, 9].
A few studies have reported on safety and delayed ischemic events when APT is discontinued after SAC. Gentric et al reported that both aspirin and clopidogrel were used more than 2 months after SAC for UICA performed using the Neuroform stent, and clopidogrel was discontinued after a mean duration of 13.6 weeks, while aspirin was discontinued after a mean duration of 32.6 weeks. However, the authors did not report on delayed ischemic events [12]. Heller et al reported on APT after treatment with the Enterprise stent; clopidogrel was discontinued after 3 months of DAPT, and then aspirin was discontinued at 6 months. Using this standard of care for APT, there was a 16% rate of delayed ischemic events [4]. Jia et al reported that after 1 month of DAPT with aspirin and clopidogrel following treatment with SAC, clopidogrel administration was stopped, and aspirin administration was stopped at 6 months; however, the authors did not report delayed ischemic events [6]. If delayed ischemic events occurred after the transition from DAPT to SAPT, DAPT was resumed. The duration of APT after SAC was not clearly defined.
APT and delayed ischemic events after coil protrusion into parent artery
One of the complications that can occur during coil embolization is coil protrusion into the parent artery [15]. Coil protrusion can result in thrombus formation in the coil and parent artery; therefore, APT and anti-coagulation therapy are both needed in the acute phase. Generally, coil protrusion of only one or two loops into the parent vessel may not cause adverse events, even without APT or anti-coagulation therapy in the chronic phase. If the coil is moving with each-pulse, it may be necessary to retrieve the coil or place a stent to control the movement [15, 16]. However, there are currently no standardized management opinions. Yamao et al reported that in a Japanese cohort of cases involving coil protrusion into the parent artery, APT was halted within 6 months in 76.5% of cases [17]. On the other hand, delayed restenosis after coil protrusion has been reported [18, 19]. In these cases, thrombus formation is presumed to be the cause of restenosis; therefore, long-term APT should be continued if a long length of coil protrudes into the parent artery. In this study, coil protrusion occurred in 4.0% (seven cases), and in five of the seven cases, APT was discontinued within 3 months. In two of the cases, SAPT was continued because a rescue stent was placed and because the patients had ischemic heart disease. No delayed ischemic events occurred in the cases with coil protrusion.
APT after coil migration to normal cerebral artery
Then a coil migrated into the cerebral artery, treatment to retrieve the coil, or rescue stent placement, and surgical removal of the coil were all considered. However, none of these approaches are easy when a small coil has migrated into the distal normal cerebral artery. In this study, the migration distances into the distal MCA were between 1.5 mm and 1 cm of the coil and all of them appeared technically difficult; fortunately, the migrations were not associated with neurological deficits and arterial flow to the coil was preserved. Therefore, DAPT was continued for 1 year, and SAPT was continued over 4 years without any ischemic events. In case of migration, if antegrade arterial flow is preserved in the migrated coil, APT should be continued, whereas, if the artery is occluded by the coil, continuous APT is not necessary.
Conclusions
After treatment of UICA with embolization, 11% of the cases required APT over 12 months due to aneurysmal factor. The reasons for long-term APT were treatment with SAC, postoperative stenosis of the small artery origin from the aneurysmal neck, coil protrusion into the parent artery, and distal migration of the coil. The use of APT after SAC is an unresolved problem, therefore, we recommend that the use of stents should be limited to the management of wide-neck or large aneurysms to prevent delayed ischemic and hemorrhagic complications. In cases that involve coil protrusion of one loop into the parent artery, there may be low risk of delayed ischemic events within the first 3 months APT.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Grant Support
None.
| References | ▴Top |
- Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331-337.
doi - Yamada NK, Cross DT, 3rd, Pilgram TK, Moran CJ, Derdeyn CP, Dacey RG, Jr. Effect of antiplatelet therapy on thromboembolic complications of elective coil embolization of cerebral aneurysms. AJNR Am J Neuroradiol. 2007;28(9):1778-1782.
doi pubmed - Fargen KM, Hoh BL, Welch BG, Pride GL, Lanzino G, Boulos AS, Carpenter JS, et al. Long-term results of enterprise stent-assisted coiling of cerebral aneurysms. Neurosurgery. 2012;71(2):239-244; discussion 244.
doi pubmed - Heller R, Calnan DR, Lanfranchi M, Madan N, Malek AM. Incomplete stent apposition in Enterprise stent-mediated coiling of aneurysms: persistence over time and risk of delayed ischemic events. J Neurosurg. 2013;118(5):1014-1022.
doi pubmed - Hwang G, Kim JG, Song KS, Lee YJ, Villavicencio JB, Suroto NS, Park NM, et al. Delayed ischemic stroke after stent-assisted coil placement in cerebral aneurysm: characteristics and optimal duration of preventative dual antiplatelet therapy. Radiology. 2014;273(1):194-201.
doi pubmed - Jia J, Lv X, Liu A, Wu Z, Li Y. Enterprise stent-assisted coiling of wide-necked intracranial aneurysms: clinical and angiographic follow-up. Interv Neuroradiol. 2012;18(4):426-431.
pubmed - Kadkhodayan Y, Rhodes N, Blackburn S, Derdeyn CP, Cross DT, 3rd, Moran CJ. Comparison of Enterprise with Neuroform stent-assisted coiling of intracranial aneurysms. AJR Am J Roentgenol. 2013;200(4):872-878.
doi pubmed - Kulcsar Z, Goricke SL, Gizewski ER, Schlamann M, Sure U, Sandalcioglu IE, Ladd S, et al. Neuroform stent-assisted treatment of intracranial aneurysms: long-term follow-up study of aneurysm recurrence and in-stent stenosis rates. Neuroradiology. 2013;55(4):459-465.
doi pubmed - Lee SJ, Cho YD, Kang HS, Kim JE, Han MH. Coil embolization using the self-expandable closed-cell stent for intracranial saccular aneurysm: a single-center experience of 289 consecutive aneurysms. Clin Radiol. 2013;68(3):256-263.
doi pubmed - Rossen JD, Chalouhi N, Wassef SN, Thomas J, Abel TJ, Jabbour PM, Kung DK, et al. Incidence of cerebral ischemic events after discontinuation of clopidogrel in patients with intracranial aneurysms treated with stent-assisted techniques. J Neurosurg. 2012;117(5):929-933.
doi pubmed - Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, Tjoumakaris S, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke. 2013;44(5):1348-1353.
doi pubmed - Gentric JC, Biondi A, Piotin M, Mounayer C, Lobotesis K, Bonafe A, Costalat V. Safety and efficacy of neuroform for treatment of intracranial aneurysms: a prospective, consecutive, French multicentric study. AJNR Am J Neuroradiol. 2013;34(6):1203-1208.
doi pubmed - Matsumoto Y, Nakai K, Tsutsumi M, Iko M, Nii K, Narita S, Eto A, et al. Onset Time of Ischemic Events and Antiplatelet Therapy after Intracranial Stent-assisted Coil Embolization. J Stroke Cerebrovasc Dis. 2014;23(4):771-777.
doi pubmed - Mocco J, Fargen KM, Albuquerque FC, Bendok BR, Boulos AS, Carpenter JS, Fiorella DJ, et al. Delayed thrombosisorstenosis following enterprise-assisted stent-coiling: is it safe? Midterm results of the interstate collaboration of enterprise stent coiling. Neurosurg. 2011;69(4):908-913.
doi pubmed - Dinc H, Kuzeyli K, Kosucu P, Sari A, Cekirge S. Retrieval of prolapsed coils during endovascular treatment of cerebral aneurysms. Neuroradiology. 2006;48(4):269-272.
doi pubmed - Sugiu K, Martin JB, Jean B, Rufenacht DA. Rescue balloon procedure for an emergency situation during coil embolization for cerebral aneurysms. Technical note. J Neurosurg. 2002;96(2):373-376.
doi pubmed - Yamao Y, Satow T, Murao K, Miyamoto S, Iihara K. [Research of postoperative complications after coil protrusions in embolization of unruptured cerebral aneurysms]. No Shinkei Geka. 2012;40(1):23-29.
pubmed - Meguro T, Sasaki T, Haruma J, Tanabe T, Muraoka K, Terada K, Hirotsune N, et al. [Arterial stenosis after coil migration in embolization of an aneurysm]. No Shinkei Geka. 2010;38(1):41-45.
pubmed - Phatouros CC, McConachie NS, Jaspan T. Post-procedure migration of Guglielmi detachable coils and Mechanical detachable spirals. Neuroradiology. 1999;41(5):324-327.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.






