Figures
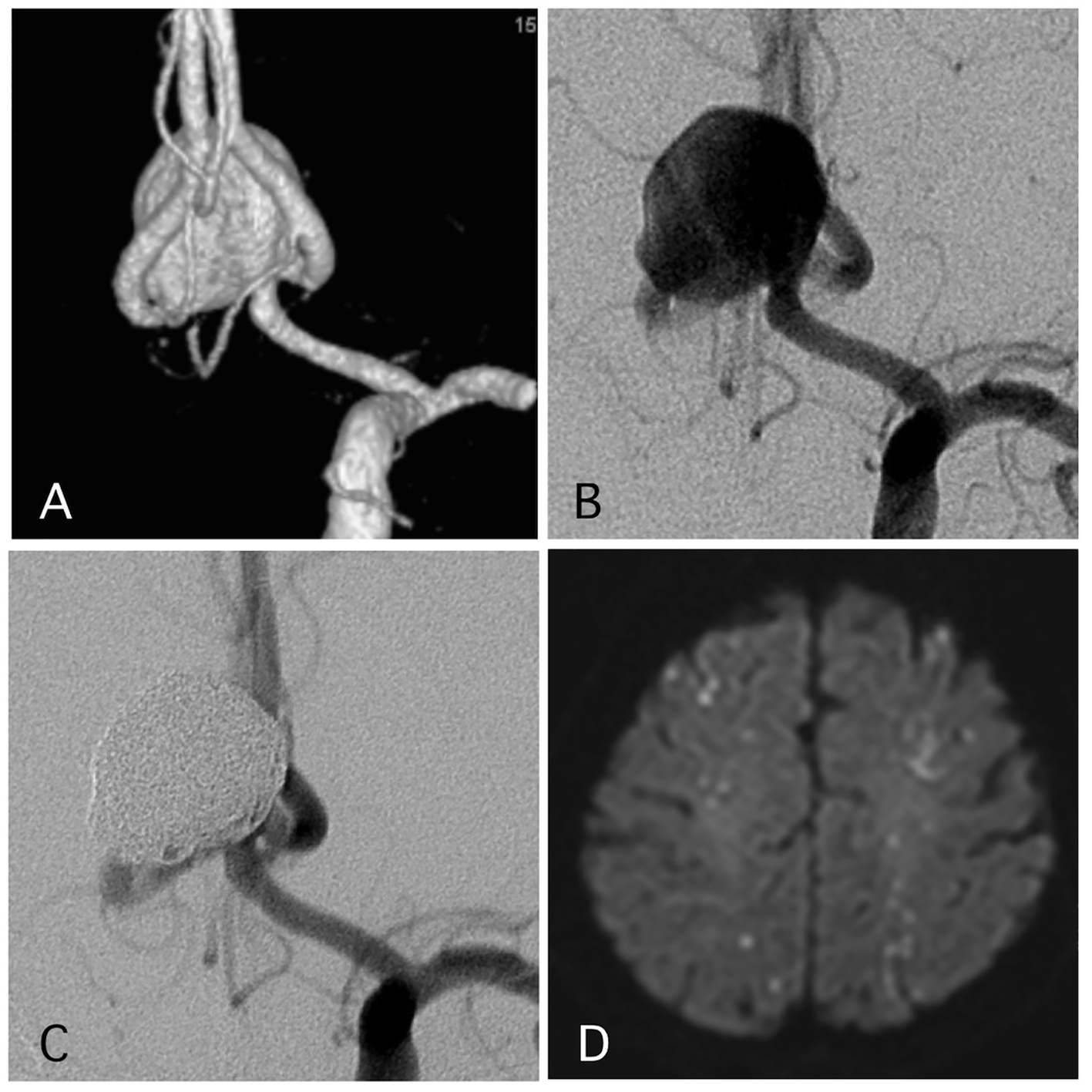
Figure 1. A representative case of a post-procedural ischemic stroke. (A) Pre-operative angiography shows a wide-neck aneurysm of the anterior communicating artery with a maximum diameter of 17 mm. (B) Pre-operative angiography. (C) Postoperative angiography reveals complete occlusion of the aneurysm with assisted stent. (D) The patient experienced transient left hemiparesis 1 day after the procedure. The antiplatelet and anti-coagulation therapies were intensified.
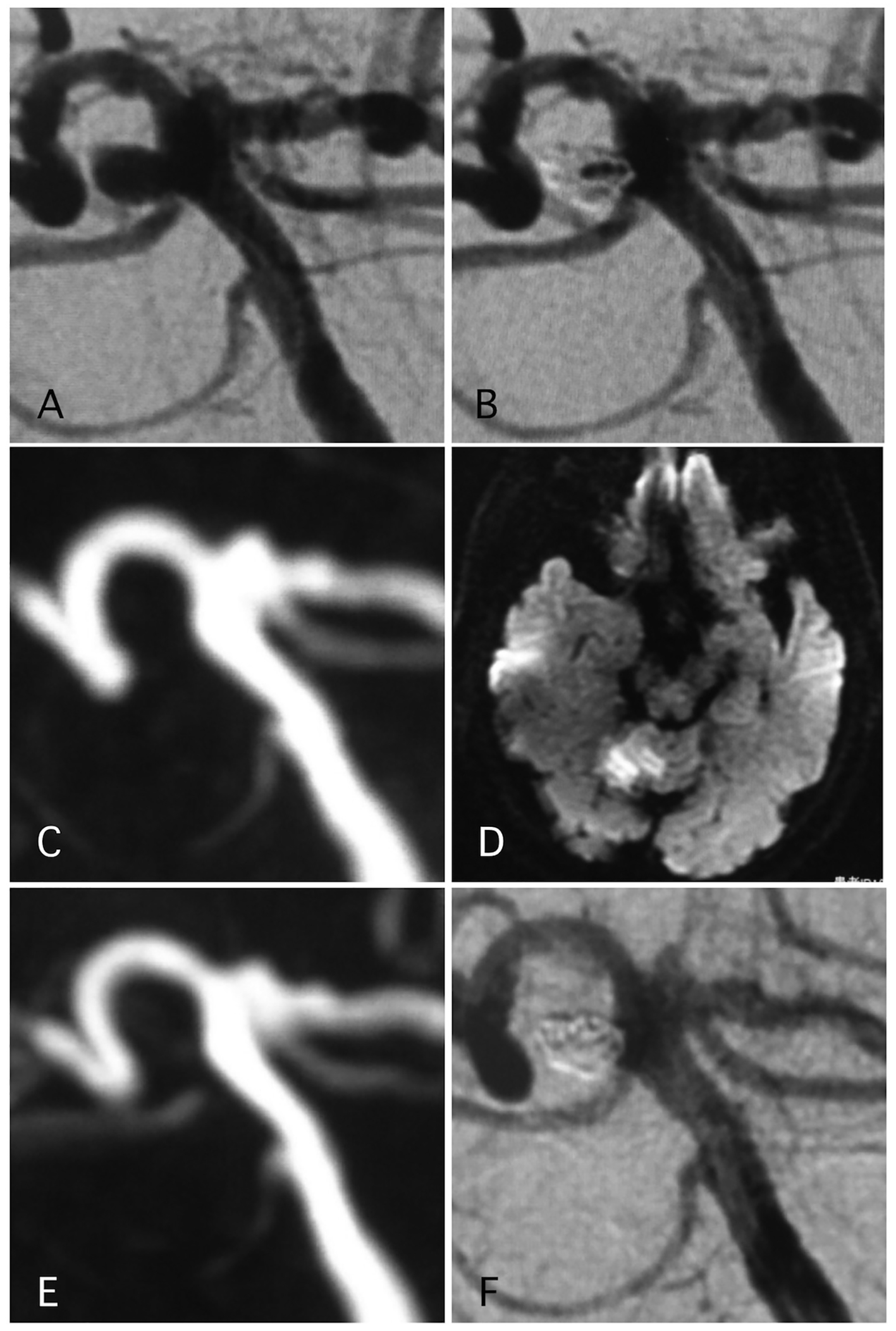
Figure 2. A representative case of post-procedural ischemic stroke. (A) Pre-operative angiography shows basilar-right superior cerebellar artery (SCA) aneurysm. (B) Postoperative angiography shows dome filling of the aneurysm. (C, D) MRI-DWI and MRA 12 days after the procedure, due to sudden trunk ataxia. Left cerebellar infarction occurred due to thrombosis of right SCA. The arrow shows stenosis of the SCA. Antiplatelet and anti-coagulation therapies were adjusted. (E) MRA 25 days after the procedure demonstrated recanalization of the SCA with weak signal of origin of right SCA. (F) Angiography 6 months after the procedure shows complete occlusion of the aneurysm and complete recanalization of right SCA.
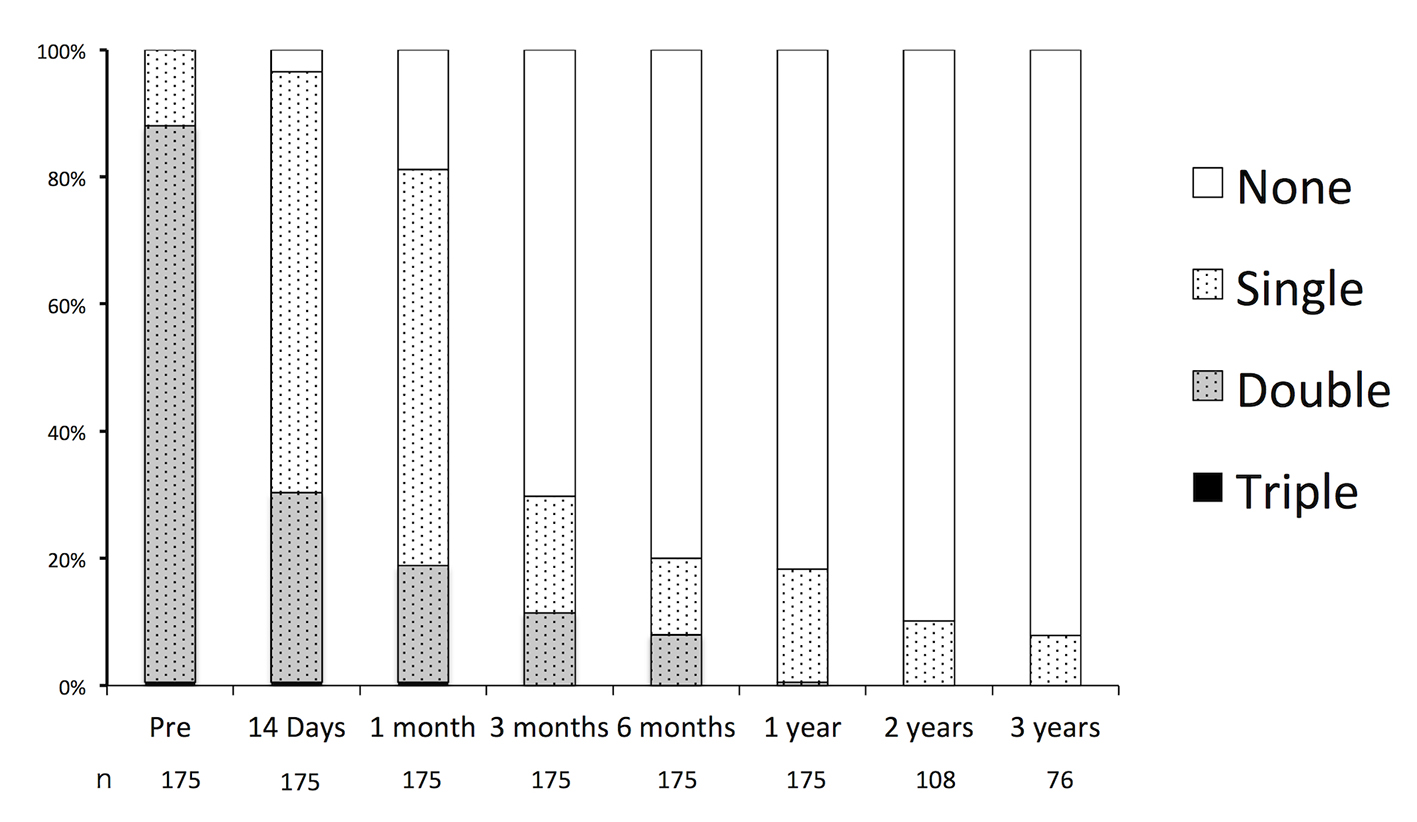
Figure 3. Durations of oral antiplatelet therapy before and after endovascular embolization of unruptured intracranial aneurysms.
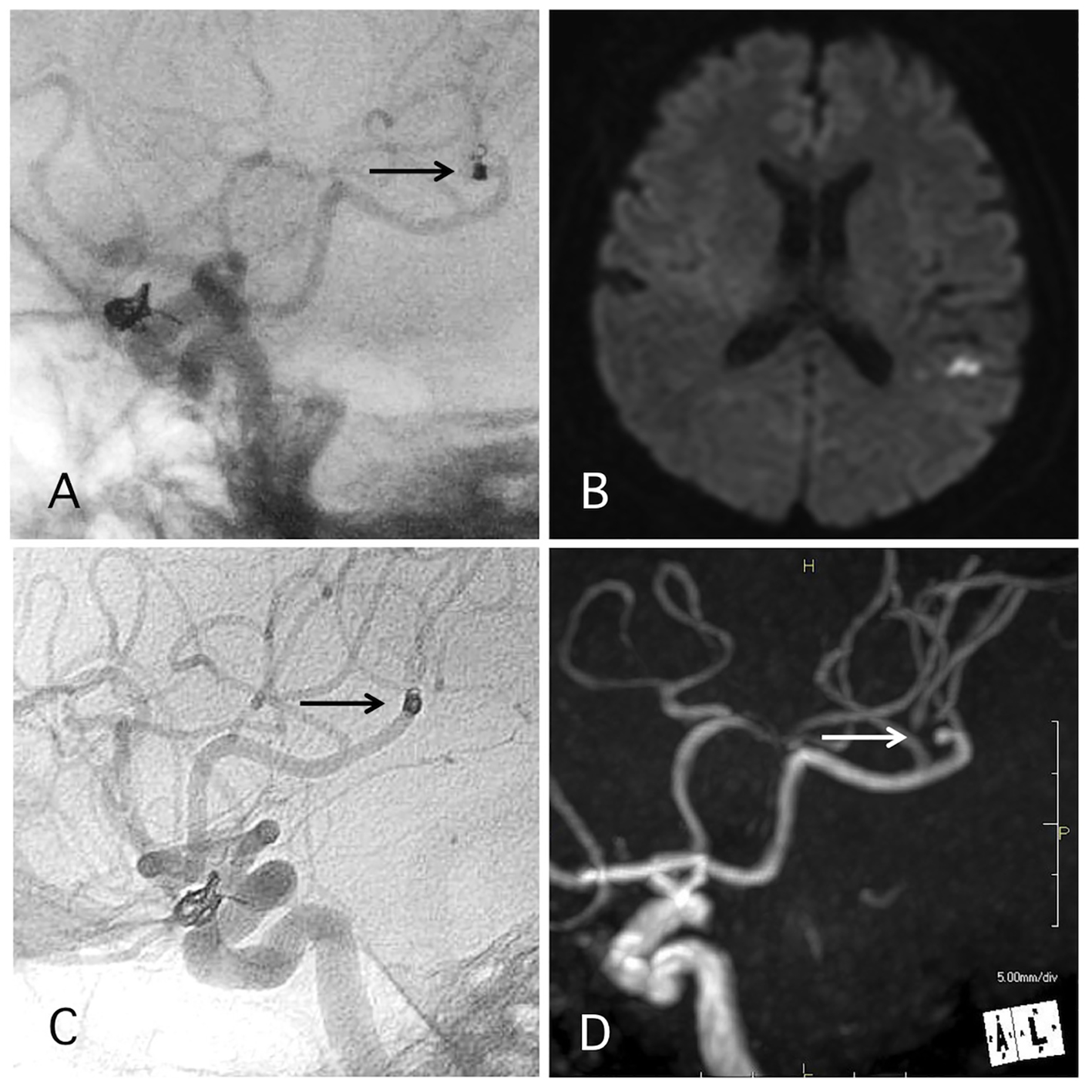
Figure 4. A representative case of post-procedural coil migration. (A) Postoperative angiography shows complete occlusion of the internal carotid artery-ophthalmic artery aneurysm with slight coil protrusion and coil migration to the middle cerebral artery (MCA). Antiplatelet therapies were continued. (B) MRI-DWI 1 day after the procedure shows cerebral infarction. (C) Angiography 1 year after the procedure shows antegrade flow of MCA through the migrated coil. (D) MRA 4 years after the procedure shows signal defect (arrow) due to the migrated coil and distal cerebral blood flow (double arrow).
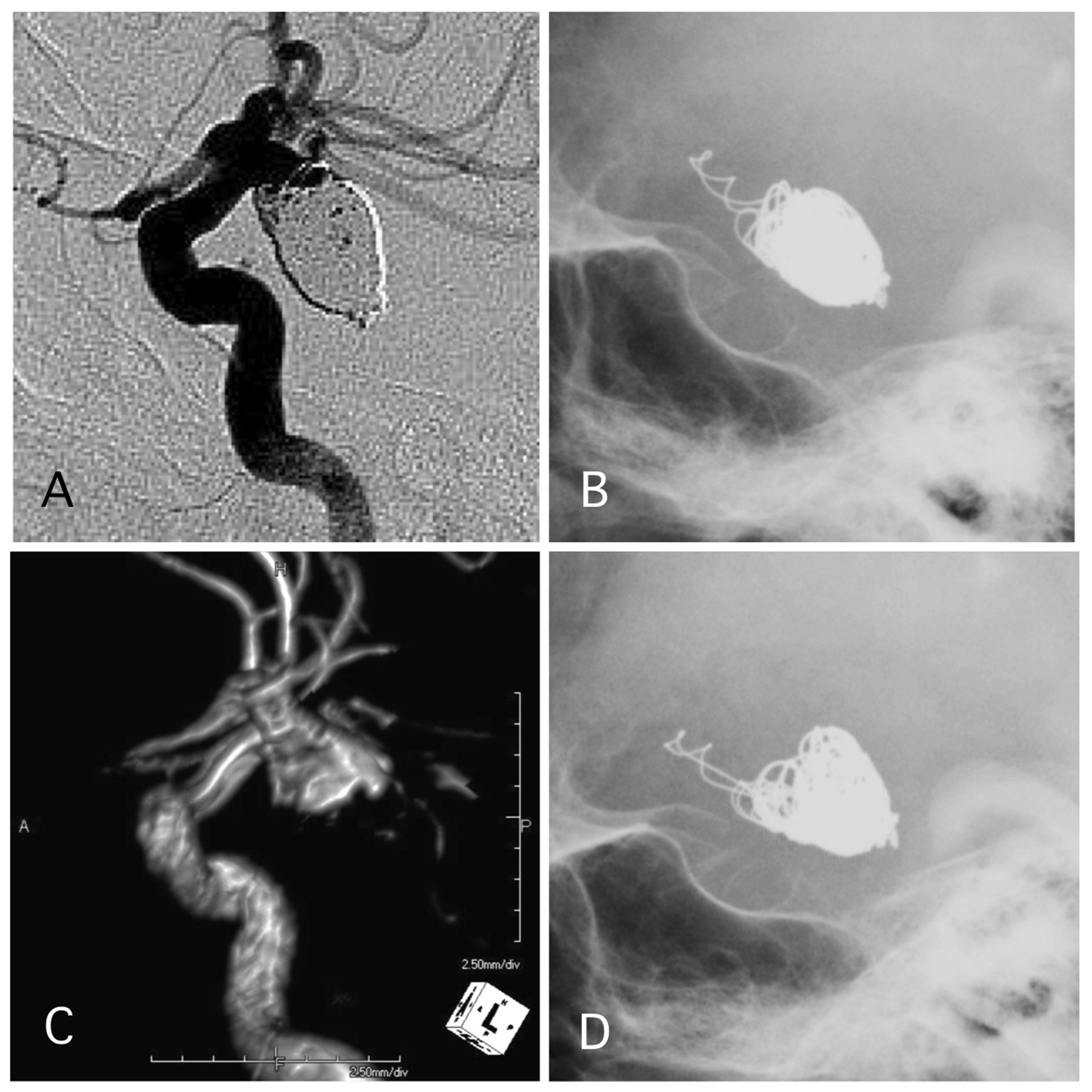
Figure 5. A representative case of post-procedural coil protrusion. (A) Postoperative angiography shows neck remnant of the internal carotid artery-posterior communicating aneurysm with no coil protrusion. (B) Skull X-ray 1 day after the procedure shows coil protrusion. Antiplatelet therapy was continued. (C) MRA 4 years after the procedure shows neck remnant of the aneurysm and no stenotic change in the internal carotid artery. (D) Skull X-ray 4 years after the procedure shows coil protrusion.
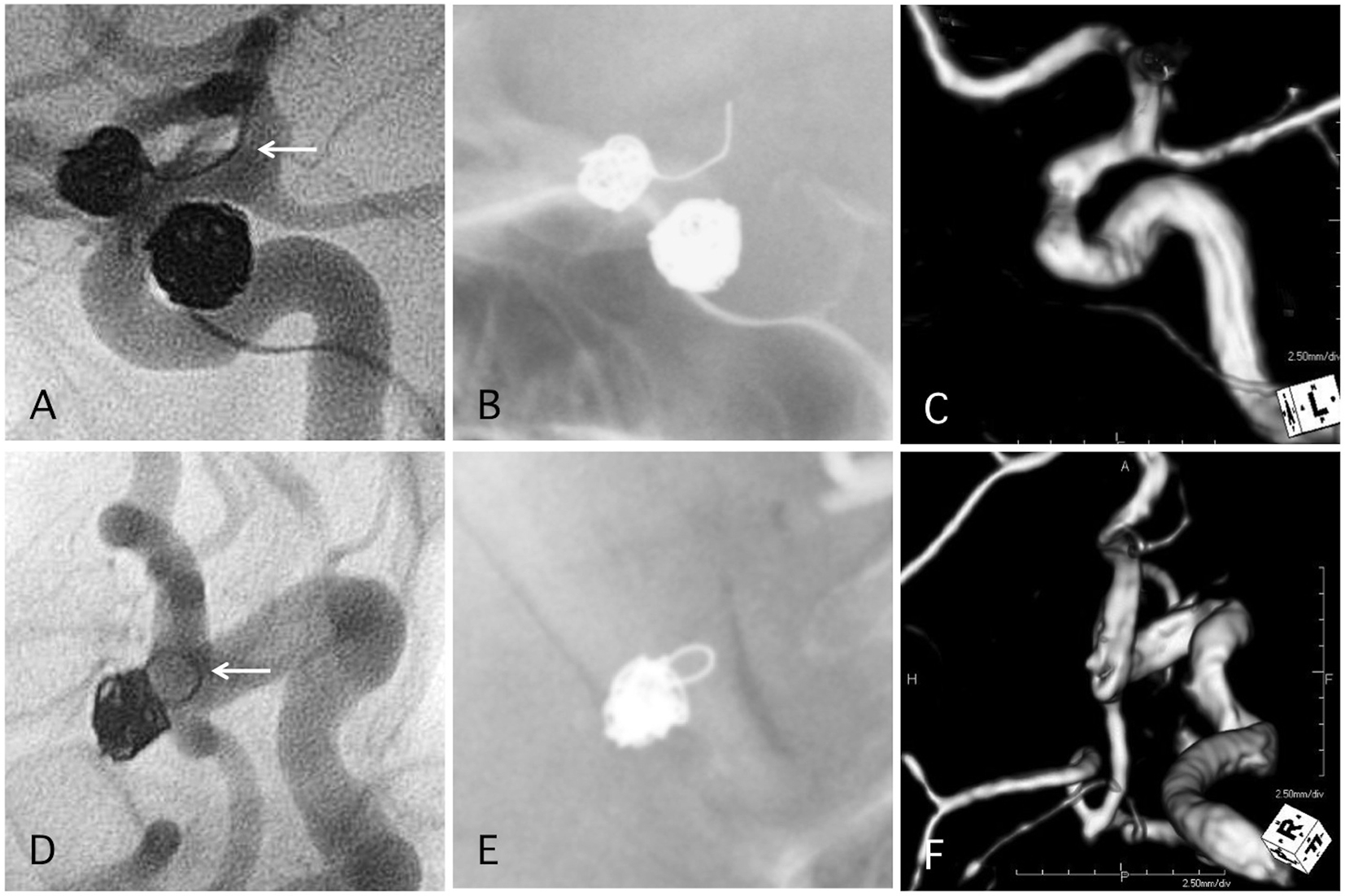
Figure 6. Two representative cases of post-procedural coil protrusion. (A) Postoperative angiography shows complete occlusion of the internal carotid artery aneurysm with one loop coil protrusion (arrow). Antiplatelet therapy is discontinued after 3months. (B) Skull X-ray 4 years after the procedure. (C) MRA 4 years after the procedure shows complete occlusion of the aneurysm and no stenotic change in the internal carotid artery. (D) Postoperative angiography shows complete occlusion of the internal carotid artery-posterior communicating aneurysm with one loop coil protrusion. Antiplatelet therapy is discontinued after 3months. (E) Skull X-ray 4 years after the procedure. (F) MRA 4 years after the procedure shows complete occlusion of the aneurysm and no stenotic change in the internal carotid artery.
Table
Table 1. Factors and Rates of Oral Antiplatelet Therapy After Endovascular Embolization of Unruptured Intracranial Aneurysms Over 1 Year
| Aneurysmal factor | 20 (62%) |
| Stent placement | 16 (50%) |
| Internal trapping | 1 (3%) |
| Postoperative stenosis of the branching artery of the aneurysm | 1 (3%) |
| Coil protrusion to parent artery | 1 (3%) |
| Coil migration to normal cerebral artery | 1 (3%) |
| Patients’ factor | 12 (38%) |
| Coronary ischemic disease | 7 (22%) |
| Cerebral ischemic disease | 5 (16%) |





