| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 4, Number 5-6, December 2014, pages 132-137
Key Role of Promoter Methylation and Inactivation of MCPH1 Gene in Brain Tumors
Fatemeh Karamia, Firoozeh Javana, Masoud Mehrazinb, Parvin Mehdipoura, c
aDepartment of Medical Genetics, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
bDepartment of Surgery, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
cCorresponding Author: Parvin Mehdipour, Department of Medical Genetics, Tehran University of Medical Sciences, Keshavarz Boulevard, Pour Sina Street, Tehran, Iran
Manuscript accepted for publication October 29, 2014
Short title: Promoter Methylation in Brain Tumors
doi: http://dx.doi.org/10.14740/jnr295e
| Abstract | ▴Top |
Background: Microcephalin (MCPH1/BIRT1) is a putative tumor suppressor gene which has recently seized great attentions in cancer studies. The present study was conducted to seek the impact of MCPH1 promoter methylation on development of brain tumors and telomere repeat length (TRL).
Methods: The brain tissue section provided from brain tumor patients and two normal brain autopsies were undergone DNA isolation. DNA samples treated with bisulfite sodium using DNA modification kit (Qiagen) were amplified in methylation specific polymerization chain reaction (MSP-PCR) confirmed by sequencing. Protein expression analysis was performed by immunofluorescence (IF) assay using antibodies against MCPH1, cyclin E and CDC25A proteins. The TRL of brain tumor patients was determined through quantitative fluorescent in situ hybridization (Q-FISH).
Results: The MCPH1 gene promoter was methylated in 96.6% of the patients consistent with protein expression pattern and the telomere statue was confirmed by low or absence of signal in tumor cells.
Conclusion: MCPH1 promoter methylation had strong association with TRL, tumor’s grade and stage (P < 0.05). TRL was meaningfully associated with grade and subtype pathology of brain tumors (P = 0.01). Further studies are required to clarify the exact role of MCPH1 gene on TRL and tumor suppression especially in brain tumors.
Keywords: MCPH1; Methylation; TRL; Protein expression; Brain tumor
| Introduction | ▴Top |
Microcephalin 1 (MCPH1) gene also known as BIRT1 was the primary gene described to be mutated in microcephaly and was mapped on 8p22-pter [1]. The BRCT repeat inhibitor of human telomerase reverse transcriptase expression (BIRT1) was reported as a repressor of TERT transcription regulating telomere repeat length (TRL) [2]. MCPH1 gene encodes for a protein with 835 amino acids and has three BRCA1 carboxyl-terminal (BRCT) domains in its structure including two C and one N-BRCT domains. It was demonstrated that the N-BRCT domain is necessary to prevent formation of premature chromosome condensation (PCC) after exposure to radiation [3]. Moreover, the indirect role of MCPH1 in homologous recombination and DNA damage response pathway has been proposed in various studies [4]. In addition, it plays pivotal roles in cell cycle and centrosome regulations. It was found that MCPH1 controls the intra-S and G2-M cell cycle checkpoints through maintaining the high levels of BRCA1 and Chk1 proteins expression. Lack of MCPH1 in microcephaly patients’ cells was associated with restricted Cdc25A degradation allowing transition of cells into the mitosis phase [5]. Absence of sufficient phosphorylated Cdk1 (at pY15) in S and G2 phases was also associated with increased level of PCC [6].
By considering these facts and downregulation of MCPH1 in different types of cancers as well as breast, prostate, ovarian and recently in oral squamous cell carcinoma (OSCC), the putative role of MCPH1 as a tumor suppressor gene is highlighted [7, 8]. However, the function of MCPH1 gene in normal brain development remains to be well elucidated in spite of defining it as one of the gene family mutated in microcephaly [9, 10]. The role of G1 phase checkpoint’s elements in neurogenesis has been determined through overexpression of cyclin D1 and E1 leading to decreased time of G1 which was associated with delayed neurogenesis in mouse embryo [11]. However, there may be an unknown interaction between G1-S with intra-S and G2-M checkpoint molecules in modulation of neurogenesis and development of brain tumors.
By considering the important role of MCPH1 gene in pathogenesis of various tumors and its elusive role in brain development, the present study was conducted to shed light on the actual character of MCPH1 gene in brain tumors. Methylation is one of the most important mechanisms proposed for inactivation of tumor suppressor genes (TSGs). Therefore, we aimed to determine the methylation statue of MCPH1 promoter gene in various types of brain tumors with different clinical and pathological characteristics. Moreover, we performed an initial protein expression analysis to show the interaction between positive cell cycle regulators including cyclin E and Cdc25A and MCPH1 as a negative regulator. In addition, due to the regulatory effect of MCPH1 on TERT transcription, telomere statue of our patients was analyzed by quantitative fluorescent in situ hybridization (Q-FISH).
| Materials and Methods | ▴Top |
Sample selection
Thirty primary brain tumor tissue samples were collected from patients who have not undergone chemotherapy or radiotherapy prior to surgery. Two normal brain tissues were taken from autopsies of two healthy individuals including one man and one woman, as control. Tissue sections were frozen in -80 °C until further processing. All the enrolled patients had filled the consent form according to the protocol of the local ethics committee.
DNA isolation
DNA was isolated from each fresh frozen tissue using QIAamp DNA Mini Kit (Qiagen) based on manufacturers’ instructions. The quality and quantity of extracted DNA samples were determined through NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and loading on agarose gel (2%).
Promoter methylation analysis
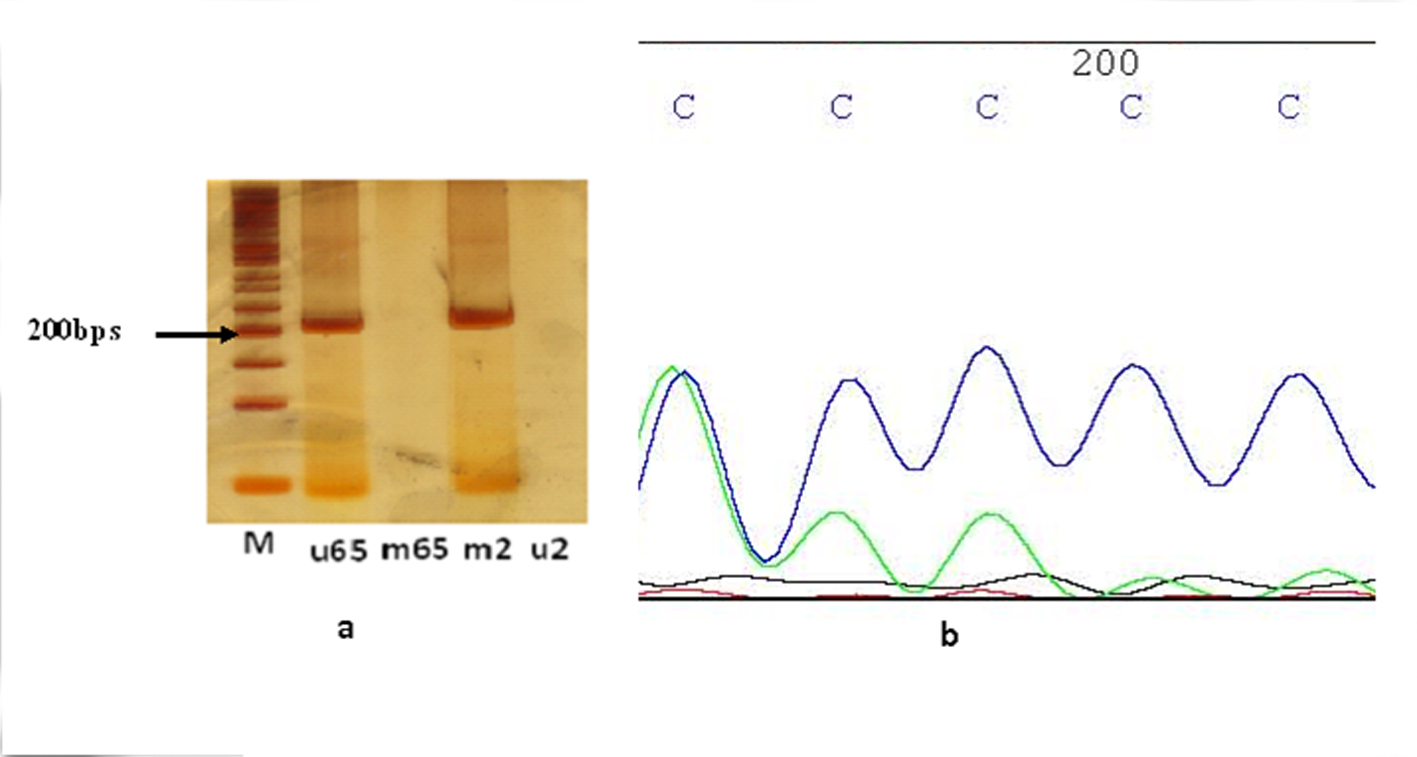
The promoter sequence of MCPH1 was retrieved from Transcriptional Regulatory Element Database (http://rulai.cshl.edu/TRED) and was assessed for the presence of CpG islands using Methprimer program (www.urogene.org/methprimer). Mehtylation statue of CpG island of MCPH1 gene was examined through methylation specific polymerase chain reaction (MSP-PCR) on isolated DNAs treated with sodium bisulfate using EpiTect Bisulfite Kit (Qiagen). MSP-PCR included two separate sets of PCR, one for detection of methylated sequences and another for unmethylated ones. Two methylated and unmethylated primer pair’s sequences were obtained from previous published paper [12]. Methylated PCR reaction contained: 10 pmol of each forward and reverse primers specific for methylated sequences, 2.5 µL of × 10 buffer, 3 mM of MgCl2, 0.2 mM of dNTP mixture and 1 U of Hot start Taq DNA polymerase (Fermentase) in addition to 100 ng of each treated genomic DNA samples adjusted with ddH2O up to final volume of 25 µL. The unmethylated reaction included the same amounts of reagents except of using 8 pmol of specific primers designed for unmethylated sequences. PCR condition for either methylated or unmethylated PCR reactions included initial denaturation in 95 °C for 5 min followed by 40 cycles of 95 °C for 30 s, annealing temperature for 30 s and extension in 72 °C for 30 s and one cycle for final extension in 72 °C for 5 min. Annealing temperatures for methylated and unmethylated PCRs were optimized at 55 °C and 50 °C respectively. The PCR products were resolved on acrylamide gel (8%) stained with AgNO3. The sizes of unmethylated and methylated PCR products were 201 and 203 bps respectively.
Immunofluorescence (IF) assay
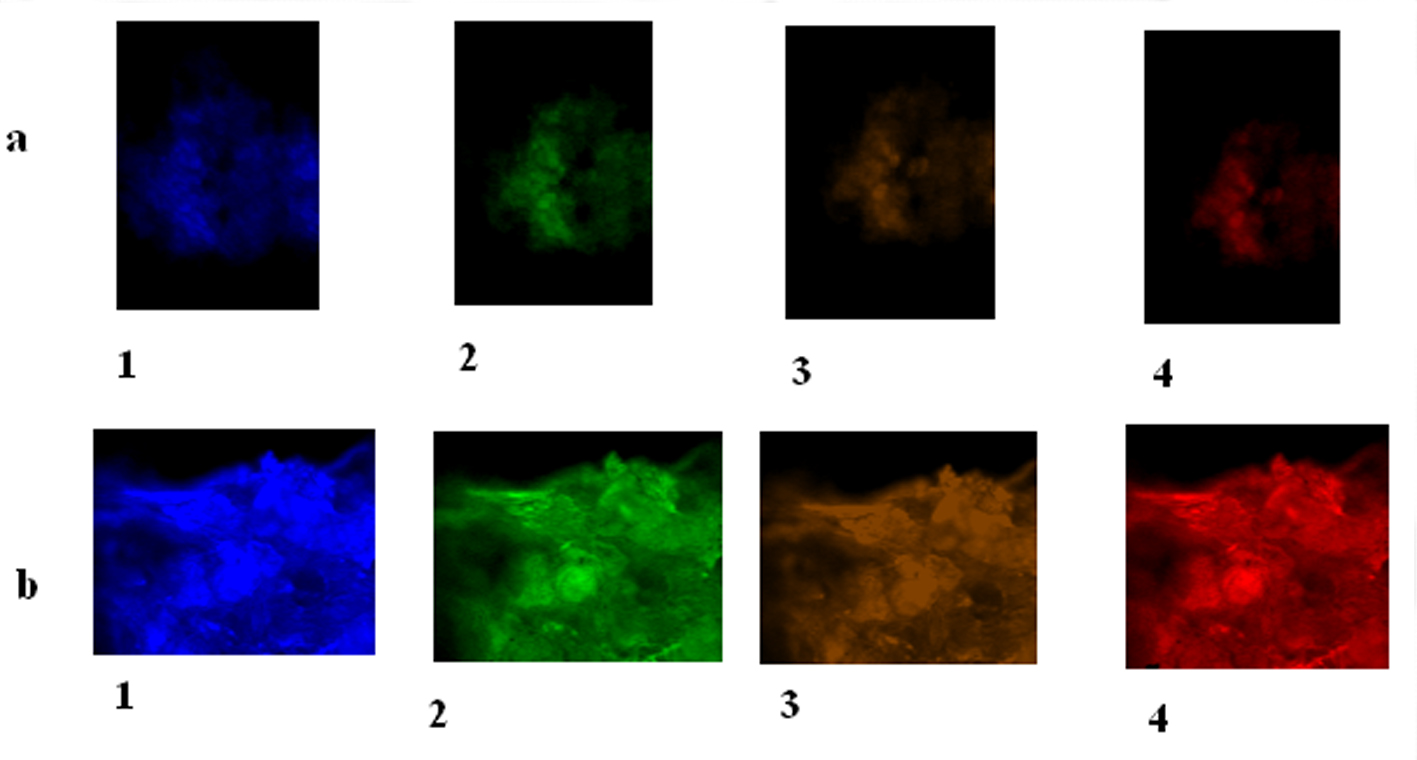
Extracted cells were stained using monoclonal mouse anti-human antibodies for MCPH1 (Abcam, UK), cyclin E (Zymed Laboratories, Invitrogen immunodetection) and Cdc25A (Sigma, USA). An average of 5,000 cells in each sample were washed twice with 16 PBS. After adding 10 mL of antibody, the mixture of cells was incubated at 4 °C for 25 min. The cells were washed by 16 PBS twice. The antibody was detected using anti-mouse IgG2b/FITC, IgG1/Pe-Cy5 (phycoerythrin-indodicarbocyanine) and IgG2a/R-pe (GENTAUR Europe, BVBA). Finally, the mode of expression was detected by the LEICA, DM RXA2-fluorescence microscope.
Assessment of telomere statue by means of Q-FISH
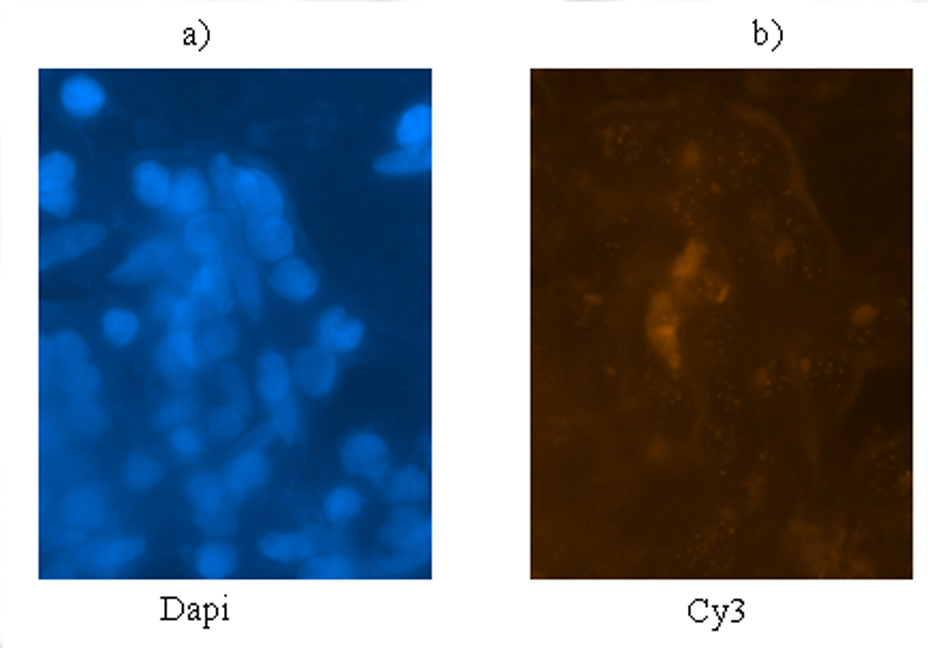
Q-FISH was used to confirm the telomere statue of brain tumor for patients whose TRL was determined using southern blotting in our previous report [13]. Q-FISH was carried out according to the instructions of Dako Company to demonstrate the alterations within the TRL by changes in fluorescent signals intensities at cellular level.
Statistical analysis
We used SPSS 16.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis of our obtained data. Chi-square, Fisher’s exact and t-tests were implicated for investigating the correlation between various variants of the patients. In all of the statistical analysis, P value of less than 0.05 was considered as significant.
| Results | ▴Top |
MSP-PCR
The results of MSP-PCR showed that the 28 out of 30 (96.6%) brain tumor samples were methylated for promoter region of MCPH1 gene while only one sample was unmethylated (3.3%) (Fig. 1a). One sample was amplified in both methylated and unmethylated PCR reactions. Among methylated tumors, based on the PCR product band intensity, one and six samples were heavily and weakly methylated respectively. Among normal tissue samples, one had methylated and the other had unmethylated MCPH1 promoter gene. The proper amplification of modified template DNAs was verified through full promoter direct sequencing using either forward methylated or unmethylated primers (Fig. 1b).
The mean age of patients was 46.16 ± 18.35 and the normal brain samples were 71 and 82 years old. The Pearson analysis did not show any correlation between age and methylation of MCPH1 promoter (P > 0.05, CI: 0.95). However, the promoter methylation of MCPH1 gene revealed to be significantly correlated with grade and stage of tumor (P = 0.01).
Protein expression analysis
The MCPH1 protein expression was very low in an astrocytoma patient which had shown methylated MCPH1 promoter while it was high in a meningioma tissue sample bearing unmethylated promoter (Fig. 2). MCPH1 protein expression was low and high in healthy controls that their MCPH1 promoter was methylated and unmethylated, respectively. Protein expression of cyclin E was low in astrocytoma and higher in meningioma. Expression of CDC25A revealed to be low in both astrocytoma and meningioma (Fig. 2).
Telomere length
As a complementary insight, status of signals intensity in brain tumor cells was assayed by Q-FISH. The results were categorized by either low intensity of signals or lack of signals in majority of cells which was compatible with low TRL (Fig. 3).
The strong association was found between TRL and either stage or grade of brain tumor (P = 0.01). This correlation was also held between subtypes of brain tumor pathologies and TRL (P = 0.01). As expected, there was positive correlation between age and TRL among all the brain tumor patients (P = 0.01). Moreover, meaningful correlation was found between TRL and methylation statue of MCPH1 promoter (P < 0.001).
| Discussion | ▴Top |
MCPH1 is one of the important genes involved in DNA repair and cell cycle checkpoint through interaction with major gatekeeper and take-care genes as well as ATM, E2F1 and BRCA1. These dual crucial roles besides its downregulation as a result of loss of heterozygosity (LOH), mutation and promoter methylation in various cancers are sufficient to call MCPH1 as a TSG [14]. Moreover, controlled TRL through suppression of TERT expression by function of MCPH1 could be considered as a vital mechanism against tumor progression. Therefore, in the present study, we sought the correlation between TRL, MCPH1 promoter methylation and pathology of different grades of malignant and benign brain tumors.
Here, it was demonstrated that promoter hypermethylation of MCPH1 gene played a pivotal role in pathogenesis of brain tumors as there was meaningful association between it and tumor grade and stage. In addition, the MCPH1 protein expression was compatible with the methylation status of its gene promoter. Given the low rate of TSG methylation in cancer, 96.6% methylation in our brain tumor samples is surprising amongst a few studies carried out on the MCPH1 gene [15]. In a recent report which was carried out on OSCC and several cell lines, promoter methylation of MCPH1 gene was determined in only 10% of tissue samples and also in SCC084 and SCC131 cell lines [8]. However, no meaningful correlation was found between clinicopathological characteristics and MCPH1 expression. In addition, in the study on promoter methylation of MCPH1 and ATM genes and their mutations in breast cancer patients, the MCPH1 promoter was methylated in 47% of breast cancer tissues [12]. Our findings are consistent with the mentioned study may indicating the importance of MCPH1 promoter methylation in either breast or brain tumors.
Moreover, no higher susceptibility to cancer in MCPH patients may imply that in contrary to hypermethylation, mutation is not sufficient to disable the major cellular functions of MCPH1 protein [16].
To the best of our knowledge, this is the first study performed to find the correlation between MCPH1 gene methylation and telomere repeat in cancer tissues especially in brain tumors compared with normal tissue specimens. In addition, this report is indicative of an interaction between two positive cell cycle regulator proteins including CDC25A and cyclin E with MCPH1 protein as an major indirect negative cell cycle protein which affects intra-S critical phases of cell cycle and probably G2-M transition. We have previously demonstrated that the expressions of both cyclin E and CDC25A proteins have been increased in 34% of primary breast cancer patients [17]. Herein, in spite of the benign nature of meningioma, cyclin E and CDC25A seem to be more expressive than in astrocytoma tumors which confirm the manner of proliferation during tumorgenesis in meningioma. This matter is not true in our patients affected with astrocytoma which is a challenging dilemma and requires complementary investigation. However, expression of MCPH1 was higher in meningioma than in astrocytoma which confirms the restricting effect of this gene, as a TSG, on tumor progression.
MCPH1 protein expression pattern was in line with its promoter methylation status confirming the direct role of methylation as a major suppressing mechanism in this gene. Moreover, we have found strong correlation between MCPH1 promoter methylation and TRL which was expected due to the confining role of MCPH1 gene expression on TRL. This strong correlation was increased with tumor grade and stage of tumor and also age of the patients. It was demonstrated that MCPH1 accompanying PNUTS is involved in modulating the TRL and protecting it against any damage happening in telomere territory through direct interaction with telomeric repeat binding factor (TRF) [18]. In contrary to previous reports [19] which have described the constant telomere length of brain tissue throughout the human life (9 - 13 kbp), TRL had been significantly decreased by age in our brain tumor patients. Furthermore, the recent finding implying on the direct positive association between TRL and brain tumor grade is in line with ours as the more tumor grade the more TRL [20].
During the initial phases of tumorgenesis by successive cell divisions, TRL gets shorter to reach the chromosomal cohesive ends. The chromosomal and genomic instability, then, provide the suitable context for tumor promotion and invasion till the short telomeres hinder further tumor proliferation after reactivation of telomerase activity [21, 22]. No meaningful association was found between MCPH1 methylation and age of patients in the present study. Accordingly, we hypothesize that in the earlier stages of tumorgenesis in brain, TRL is short which is consistent with weakly methylated or full unmethylated MCPH1 promoter. Methylation of MCPH1 promoter in higher grade of brain tumor may help TERT and telomerase escape from the control of MCPH1 to be expressed. Testing and evaluating this hypothesis warrants larger sample size and assessment of up- and down-stream genes within the molecular pathways in which MCPH1 gene is active.
Taken together, promoter methylation of MCPH1 gene demonstrated the strong correlation with various types of brain tumor’s grade, stage and TRL. The potential of drugs such as 5-azacytidine in reversing the methylation statue of promoter sequences was recently approved in treatment of leukemia and is currently under the trials to be used in management of solid tumors in future [23]. Therefore, identification of promoter methylation as the main inactivation mechanism of TSG constitutes the major part of cancer genetics. Due to the high promoter methylation of MCPH1 gene in brain tumor, further expression assay is required which is as our ongoing project. However, the results will facilitate to evaluate the possibility of using it as an exclusive diagnostic, prognostic and therapeutic tool in brain tumors. Moreover, to clarify the precise developmental role of MCPH1 gene in brain tumor, deeper complementary study is essential.
As an initial statement, the promoter methylation revealed to be, critically, involved in development of brain tumors.
Acknowledgement
This is to acknowledge the nursery and surgery teams in Shariati Hospital, and also the patients for their supports.
Conflict of Interests
There is no conflict of interest among all of the authors of this manuscript.
Abbreviations
TRL: telomere repeat length; MSP-PCR: methylation specific polymerization chain reaction; IF: immunofluorescence; Q-FISH: quantitative fluorescent in situ hybridization; MCPH1: microcephalin 1; BRCT: BRCA1 carboxyl-terminal; PCC: premature chromosome condensation; BIRT1: BRCT repeat inhibitor of human telomerase reverse transcriptase expression; TSGs: tumor suppressor genes; LOH: loss of heterozygosity; OSCC: oral squamous cell carcinoma
| References | ▴Top |
- Jackson AP, McHale DP, Campbell DA, Jafri H, Rashid Y, Mannan J, Karbani G, et al. Primary autosomal recessive microcephaly (MCPH1) maps to chromosome 8p22-pter. Am J Hum Genet. 1998;63(2):541-546.
doi pubmed - Lin SY, Elledge SJ. Multiple tumor suppressor pathways negatively regulate telomerase. Cell. 2003;113(7):881-889.
doi - Brown JA, Bourke E, Liptrot C, Dockery P, Morrison CG. MCPH1/BRIT1 limits ionizing radiation-induced centrosome amplification. Oncogene. 2010;29(40):5537-5544.
doi pubmed - Liang Y, Gao H, Lin SY, Peng G, Huang X, Zhang P, Goss JA, et al. BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet. 2010;6(1):e1000826.
doi pubmed - Brunk K, Vernay B, Griffith E, Reynolds NL, Strutt D, Ingham PW, Jackson AP. Microcephalin coordinates mitosis in the syncytial Drosophila embryo. J Cell Sci. 2007;120(Pt 20):3578-3588.
doi pubmed - Alderton GK, Galbiati L, Griffith E, Surinya KH, Neitzel H, Jackson AP, Jeggo PA, et al. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling. Nat Cell Biol. 2006;8(7):725-733.
doi pubmed - Pan S, Dong Q, Sun LS, Li TJ. Mechanisms of inactivation of PTCH1 gene in nevoid basal cell carcinoma syndrome: modification of the two-hit hypothesis. Clin Cancer Res. 2010;16(2):442-450.
doi pubmed - Venkatesh T, Nagashri MN, Swamy SS, Mohiyuddin SM, Gopinath KS, Kumar A. Primary microcephaly gene MCPH1 shows signatures of tumor suppressors and is regulated by miR-27a in oral squamous cell carcinoma. PLoS One. 2013;8(3):e54643.
doi pubmed - Richardson J, Shaaban AM, Kamal M, Alisary R, Walker C, Ellis IO, Speirs V, et al. Microcephalin is a new novel prognostic indicator in breast cancer associated with BRCA1 inactivation. Breast Cancer Res Treat. 2011;127(3):639-648.
doi pubmed - Rai R, Dai H, Multani AS, Li K, Chin K, Gray J, Lahad JP, et al. BRIT1 regulates early DNA damage response, chromosomal integrity, and cancer. Cancer Cell. 2006;10(2):145-157.
doi pubmed - Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5(3):320-331.
doi pubmed - Bhattacharya N, Mukherjee N, Singh RK, Sinha S, Alam N, Roy A, Roychoudhury S, et al. Frequent alterations of MCPH1 and ATM are associated with primary breast carcinoma: clinical and prognostic implications. Ann Surg Oncol. 2013;20(Suppl 3):S424-432.
doi pubmed - Kheirollahi M, Mehrazin M, Kamalian N, Mehdipour P. Alterations of telomere length in human brain tumors. Med Oncol. 2011;28(3):864-870.
doi pubmed - Yang SZ, Lin FT, Lin WC. MCPH1/BRIT1 cooperates with E2F1 in the activation of checkpoint, DNA repair and apoptosis. EMBO Rep. 2008;9(9):907-915.
doi pubmed - Zemliakova VV, Zhevlova AI, Strel'nikov VV, Liubchenko LN, Vishnevskaia Ia V, Tret'iakova VA, Zaletaev DV, et al. [Abnormal methylation of several tumor suppressor genes in sporadic breast cancer]. Mol Biol (Mosk). 2003;37(4):696-703.
- O'Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nat Rev Genet. 2006;7(1):45-54.
doi pubmed - Mehdipour P, Pirouzpanah S, Sarafnejad A, Atri M, Shahrestani ST, Haidari M. Prognostic implication of CDC25A and cyclin E expression on primary breast cancer patients. Cell Biol Int. 2009;33(10):1050-1056.
doi pubmed - Kim H, Lee OH, Xin H, Chen LY, Qin J, Chae HK, Lin SY, et al. TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat Struct Mol Biol. 2009;16(4):372-379.
doi pubmed - Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220(1):194-200.
doi pubmed - La Torre D, Conti A, Aguennouz MH, De Pasquale MG, Romeo S, Angileri FF, Cardali S, et al. Telomere length modulation in human astroglial brain tumors. PLoS One. 2013;8(5):e64296.
doi pubmed - Londono-Vallejo JA. Telomere instability and cancer. Biochimie. 2008;90(1):73-82.
doi pubmed - Shay JW, Wright WE. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett. 2010;584(17):3819-3825.
doi pubmed - Chik F, Machnes Z, Szyf M. Synergistic anti-breast cancer effect of a combined treatment with the methyl donor S-adenosyl methionine and the DNA methylation inhibitor 5-aza-2'-deoxycytidine. Carcinogenesis. 2014;35(1):138-144.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.