| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Letter to the Editor
Volume 9, Number 1-2, March 2019, pages 18-20
Occam’s Razor Is Not Always True: Intracranial Tumor Mimicking Silent Infarction in a Patient With Previous Cerebral Infarction
Halil Ondera, d, Ibrahim Akkurtb, Guven Arslana, Sahin Hanaliogluc
aNeurology Clinic, Yozgat City Hospital, Yozgat, Turkey
bNeurosurgery Clinic, Yozgat City Hospital, Yozgat, Turkey
cDiskapi Yildirim Beyazit Training and Research Hospital, Ankara, Turkey
dCorresponding Author: Halil Onder, Neurology Clinic, Yozgat City Hospital, Yozgat, Turkey
Manuscript submitted November 29, 2018, accepted December 26, 2018
Short title: Intracranial Tumor Mimicking Silent Infarction
doi: https://doi.org/10.14740/jnr515
| To the Editor | ▴Top |
A 67-year-old male patient was admitted with a complex partial seizure attack characterized by left-sided convulsion lasting 10 min. On neurological examination at admission, the patient was cooperative and orientated, but a mild confusion was recognized. A slight left-sided paralysis was present; other examinations were within normal limits. Upon history taking, it was learned that he had had a left medullary stroke 3 years ago and had been receiving aspirin 300 mg regularly since then. Routine hematological and biochemical tests were unremarkable. Cranial computerized tomography (CT) showed mild hypodensity in the right insula, parietal subcortical region (Fig. 1). Cranial diffusion-weighted imaging (DWI) showed a lesion in the right insula-frontal lobe which was isointense on DWI and hyperintense on apparent diffusion coefficient (ADC) maps, suggesting chronic infarction (Fig. 2). It was learned (from the hospital records) that cranial magnetic resonance imaging (MRI), performed 3 years ago at hospitalization, had shown left medullary ischemic lesion, but no lesion corresponding to this region (right frontal). During the interval period up to date, the patient had no neurological symptom or history of recurrent stroke. Taken together, we evaluated the newly recognized lesion in the right insula as silent infarction and demanded further etiological investigations for recurrent stroke. Of note, we associated the left-sided mild paralysis with Todd’s paralysis which totally resolved in the following several hours interval. Brain/neck tomography angiography, echocardiography, and rhythm Holter examinations were within normal limits. Tumor markers (carcinoembryonic antigen, CA 125, CA 19-9, CA 15-3, and prostate-specific antigen) were within normal ranges. Stroke was evaluated as a cryptogenic stroke and clopidogrel was added to the treatment. Routine electroencephalogram (EEG) showed paroxysmal activity in the right frontotemporal lobe. Levetiracetam 2 × 500 mg was initiated for epileptic seizure. However, the patient was readmitted to the emergency service with secondary generalized convulsive seizures 3 months after discharge. Cranial tomography showed an edematous lesion in the same region this time. Hence, contrast-enhanced brain MRI was performed which yielded the diagnosis of the intracranial tumor (Fig. 3). Dexamethasone therapy was initiated and levetiracetam dosage was increased up to 2,000 mg daily. After recovery to pre-morbid state, the patient was referred to a neuro-oncology center for administration of further therapies.
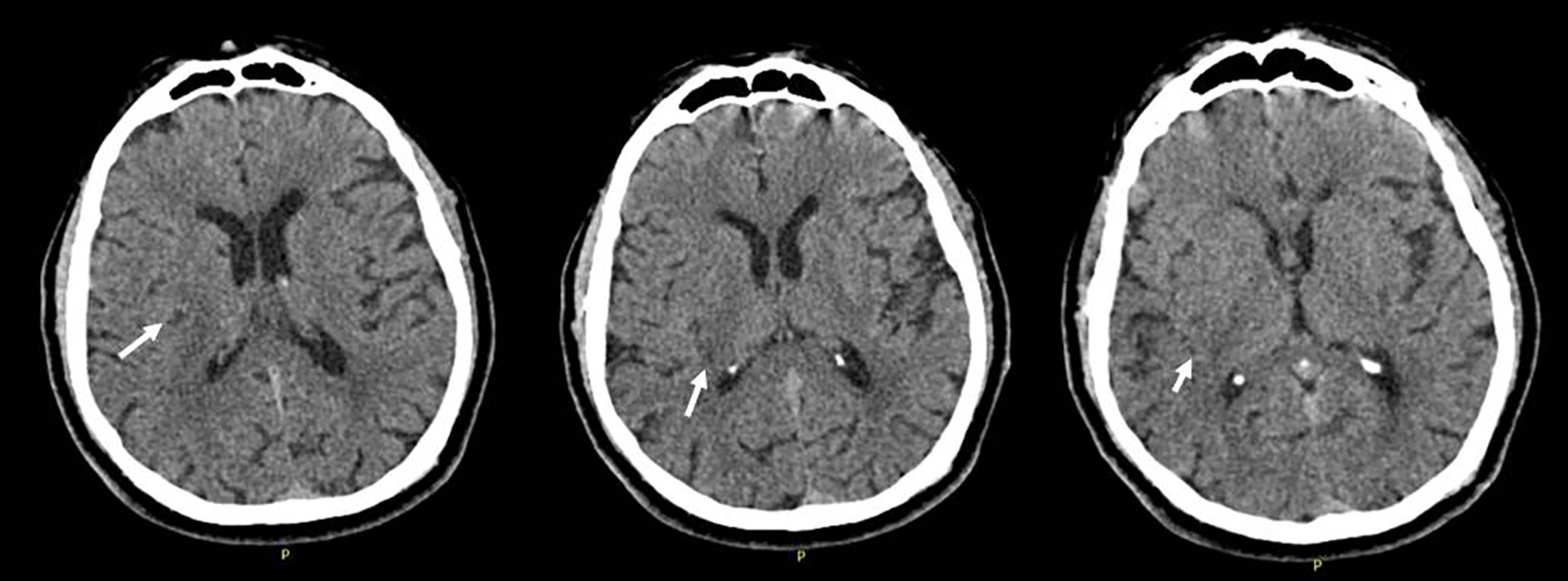 Click for large image | Figure 1. Cranial computerized tomography (CT) performed at initial admission, showing mild hypodensity in the right insula, parietal subcortical region. |
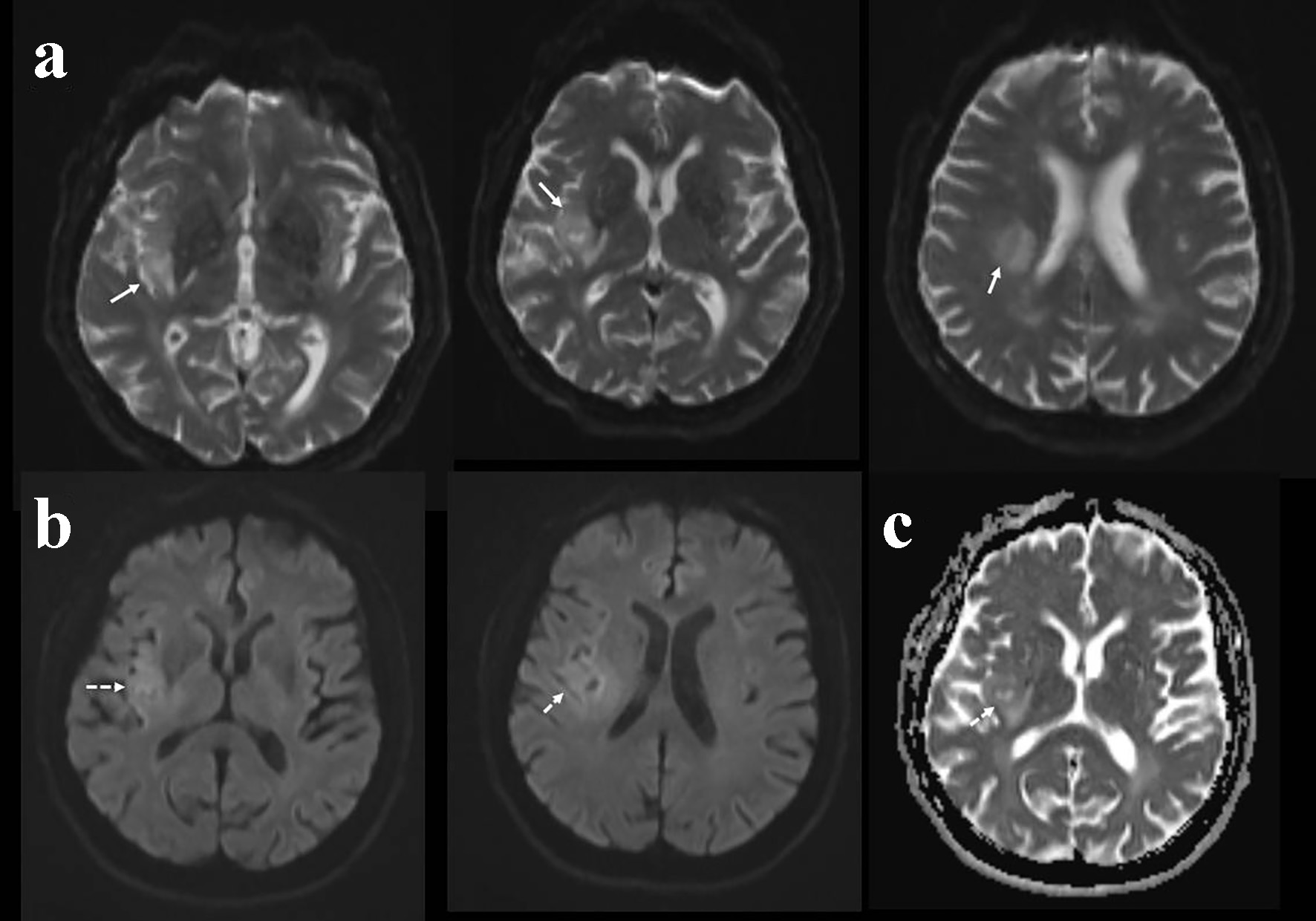 Click for large image | Figure 2. Diffusion-weighted imaging (DWI) at initial admission showing hyperintensity in the right insular and parietal region in the unmodified DWI sequences (a), mild hyperintensity in the DWI sequences considered as T2 shine effect (b). Apparent diffusion coefficient (ADC) sequences showing hyperintense signals in the corresponding region suggesting its chronic nature (c). |
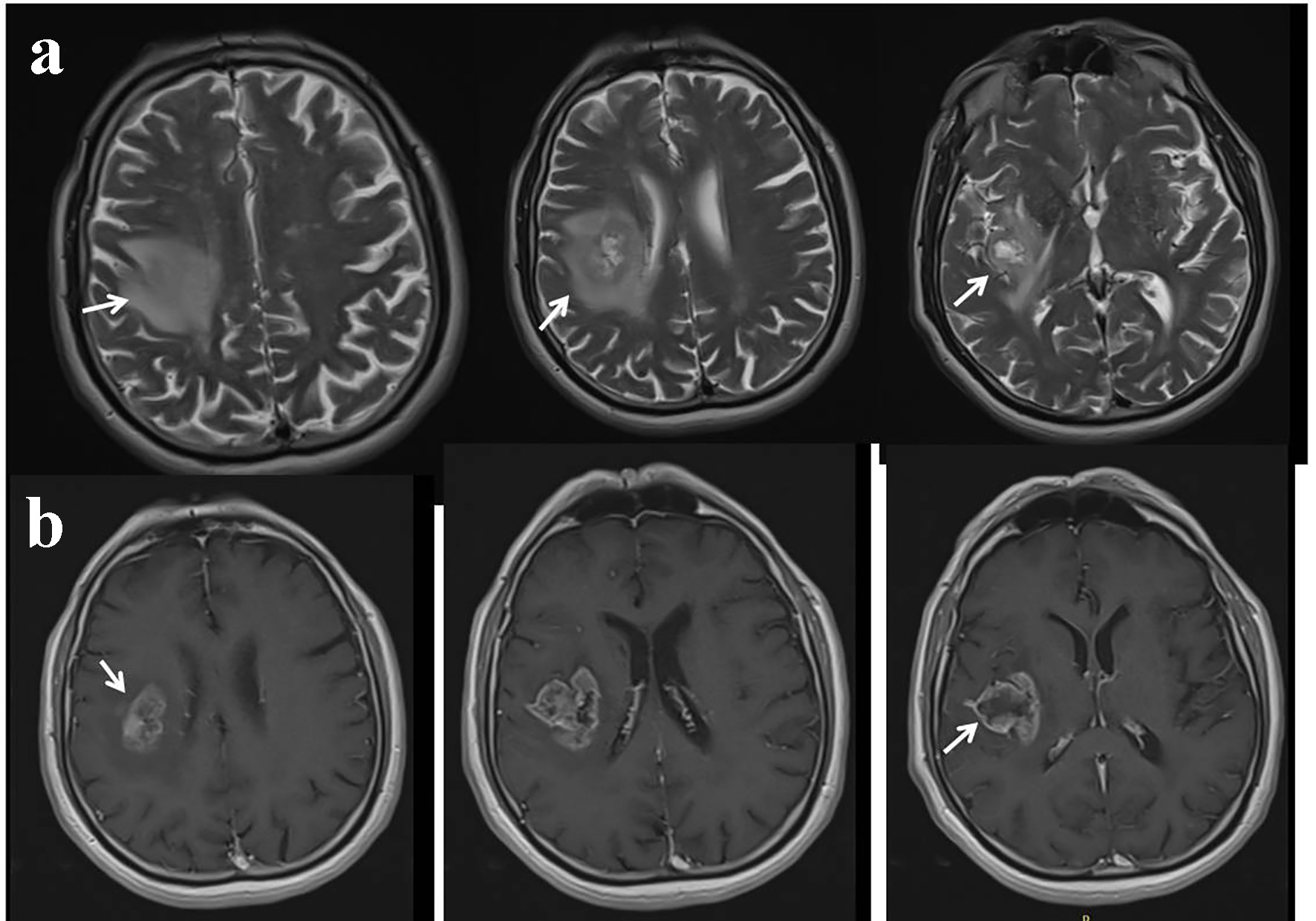 Click for large image | Figure 3. Contrast-enhanced cranial magnetic resonance imaging (MRI) performed as second admission. T2 sequences showing edematous lesion in the right frontoparietal lobe (a). T1 post-contrast sequences showing significant contrast enhancement (b). |
Occasionally, a brain tumor can present with stroke-like symptoms. The clinical history of an acute onset of symptoms and matching medical history is usually suggestive of stroke. On the other hand, silent infarction which can be defined as cerebral infarctions that are observed in the radiological imaging in the absence of corresponding clinical symptomatology may further complicate the diagnostic process. Herein, we present a rare patient with an intracranial tumor which was initially diagnosed as silent infarction. At admission to the emergency service, he had a mild paresis in the left side that was evaluated clinically as Todd’ paralysis. The total recovery of the paralysis in the acute phase and abnormal EEG findings also confirmed the diagnosis. Hence, he was discharged on dual antiplatelet therapy and anti-epileptic therapy. However, the clinical recurrence of the seizure and edematous appearance in the newly recorded brain CT led to interrogation of the initial diagnosis. Finally, contrast-enhanced MRI findings provided the diagnosis of the intracranial tumor. Nevertheless, we would also like to state that the major limitation of this case was the absence of pathological investigations which restricted to totally exclude a possible tumefactive lesion in this patient. However, progression of the lesion in the interval period and symptoms of elderly onset were also atypical features for a tumefactive lesion. Via the presentation of this patient, we emphasize the importance of detailed interrogation of the clinical and radiological findings before making the diagnosis of silent infarction. The incidence of seizure in patients with brain tumors is considerably high which was estimated to be approximately 30% [1]. On the other hand, its incidence in the late phase of stroke was reported to be 1.12 per 100 person-years [2]. Taken together, manifestation of a seizure may be a crucial clue in the clinical practice which may suggest a tumoral lesion other than ischemic origin as it can present rather rarely in post-stroke patients. However, we also think that our radiological evaluation process of DWI-based interpretation may constitute the major handicap in this patient. Based on this experience and related literature, we emphasize to conduct full brain MRI exam before making the diagnosis of chronic infarction, particularly in the subgroup without a history of corresponding, clinical symptomatology. We think that history of the vascular risk factors (hypertension and hyperlipidemia) and previous ischemic stroke might have been efficient in the imprecise evaluation processes at first admission (based on the DWI in the absence of conventional brain MRI), resulting in the initial misdiagnosis of silent infarction. Ergo, we would also like to emphasize the importance of being very careful during the diagnostic processes of patients presenting with a new neurological clinic and history of neurological comorbidities. Therefore, we preferred to present this remarkable case as a smart sample leading to the interrogation of the rationality of “Occam’s Razor”.
“Occam’s Razor” is a principle in science and philosophy, much applied in medicine, that one should try to account for an observed phenomenon in the simplest possible way and should not look for multiply explanations of its different aspects.
Acknowledgments
None.
Funding
None.
Conflict of Interest
None.
| References | ▴Top |
- Morris HH, Estes ML, Gilmore R, Van Ness PC, Barnett GH, Turnbull J. Chronic intractable epilepsy as the only symptom of primary brain tumor. Epilepsia. 1993;34(6):1038-1043.
doi pubmed - Chan L, Hu CJ, Fan YC, Li FY, Hu HH, Hong CT, Bai CH. Incidence of poststroke seizures: A meta-analysis. J Clin Neurosci. 2018;47:347-351.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
