| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 1, Number 4, October 2011, pages 153-160
Serbian Validation of the Individualized Neuromuscular Quality of Life Questionnaire (INQoL) in Adults With Myotonic Dystrophy Type 1
Stojan Perica, c, Valeria Sansoneb, Dragana Lavrnica, Giovanni Meolab, Ivana Bastaa, Marina Miljkovica, Vidosava Rakocevic-Stojanovica
aNeurology Clinic, Clinical Centre of Serbia, School of Medicine, University of Belgrade, Belgrade, Serbia
bDepartment of Neurology, University of Milan, IRCCS Policlinico San Donato, Milan, Italy
cCorresponding author: Stojan Peric
Manuscript accepted for publication September 27, 2011
Short title: Serbian INQoL in myotonic dystrophy type 1
doi: https://doi.org/10.4021/jnr54w
| Abstract | ▴Top |
Background: To validate Individualized Neuromuscular Quality of Life Questionnaire (INQoL) in Serbian patients with myotonic dystrophy type 1 (DM1).
Methods: This study included 102 patients with adult onset DM1. Validation included reliability analysis (internal consistency, reproducibility), content-related validity (psychometric evaluation, construct-related validity, criterion-related validity) and concurrent validity.
Results: The internal consistency of the Serbian version of INQoL was excellent (Cronbach’s alpha 0.864 - 0.961). Test-retest reliability satisfied the requested level (intraclass correlation coefficient 0.713 - 0.979). Item internal consistency and discriminant validity were excellent. INQoL scores were significantly affected by age of patients, duration of disease and severity of muscular impairment (P < 0.01), slightly affected by education (P < 0.05) and not related to gender (P > 0.05). Correlation between INQoL scales and comparable SF-36 domains was significant (P < 0.01) but INQoL also registered locking and body image omitted by SF-36.
Conclusion: Serbian version of INQoL is reliable and valid quality of life (QoL) measure for patients with DM1, able to capture disease specific issues usually omitted by generic questionnaires.
Keywords: INQoL; Myotonic dystrophy type 1; Quality of life; Validation
| Introduction | ▴Top |
Myotonic dystrophy type 1 (DM1) is the most common muscular dystrophy in adults and can affect muscular, cardiac, ocular, endocrine, respiratory, gastrointestinal and central nervous system [1]. Progressive multi-organ involvement leads to increasing disability and restricted social participation [2]. Measuring quality of life (QoL) in this chronic and incurable disorder is essential [3] since it allows to target the management of such patients. However, there are very few studies that have specifically assessed QoL in DM1 [4, 5]. Generic QoL questionnaires like SF-36 have been used in DM1 patients to assess the influence of some lesser recognized symptoms such as fatigue, daytime sleepiness and pain on QoL [6-8] and as a secondary outcome measure in clinical trials [9, 10]. The use of generic questionnaires is acceptable in patients with neuromuscular disorders [3], but they can capture some issues superfluous to those with muscle disorders and omit some important issues which may instead be captured by disease or symptom specific QoL questionnaire.
The Individualized Neuromuscular Quality of Life questionnaire (INQoL) is a muscle disease specific QoL measure which has been developed and validated in the United Kingdom on a heterogeneous group of patients with neuromuscular disorders [11]. INQoL has also been validated in Italy confirming that INQoL is a reliable, valid and practical measure for QoL assessment able to capture issues specifically relevant to the muscle condition [12]. These studies suggested that validity of INQoL should be extended to other countries using larger and more homogenous diagnostic groups [11, 12].
The aim of this study was to translate and validate INQoL for use in Serbian patients with DM1.
| Materials and Methods | ▴Top |
Patients with adult onset DM1 were consecutively recruited from the outpatient clinics of the Department of Neuromuscular Disorders of the Neurology Clinic, Clinical Centre of Serbia in the period from July 2009 until October 2010. The diagnosis of DM1 was confirmed by gene analysis (CTG between 200-800 repeats). Test-retest reliability of INQoL was assessed by administering it twice 10 days apart in first 30 subjects recruited. The 10 day interval was considered short enough to minimize actual changes in DM1 condition but long enough to reduce recall. All patients gave their informed consent to participate in the study and the study was approved by the Ethical Board of the Neurology Clinic. Patients were assessed using Muscular Impairment Rating Scale (MIRS) and they also completed the SF-36 and INQoL.
The Muscular Impairment Rating Scale (MIRS) was used to rate severity of muscular involvement in DM1 [13]. The MIRS is an ordinal five-point rating scale, established in accordance with the clinically recognized distal to proximal progression of the muscular involvement in DM1, and based partly on a manual muscle testing of 11 muscle groups. It is a quick, simple, and reliable measurement of muscular impairment in DM1. The MIRS is useful to monitor major stages of DM1 progression, to study the natural history of the disease, and to identify homogeneous groups of patients for clinical trials [13].
All patients were also assessed with SF-36, Serbian version [14], which is the most widely used patient-based health-related generic questionnaire. It was also proved to be valid for use in patients with neuromuscular disorders [3]. The SF-36 is a multi-item scale that assesses eight health concepts: limitations in physical activities (PF), limitations in usual role activities due to physical problems (RP), bodily pain (BP), general health perception (GH), vitality (VT), limitations in social activities (SF), limitations in usual role activities due to emotional problems (RE) and general mental health (MH). Each of eight domains is scored on a 0-100 scale, with a higher score indicating a better health-related QoL. In addition, it is possible to calculate physical composite score (PCS) consisting of PF, RP, BP and GH domains, mental composite score (MCS) consisting of VT, SF, RE and MH domains, as well as total SF-36 score. All scores are given in a 0-100 point scale.
Patients completed INQoL [11]. INQoL consists of 45 questions within 10 sections. Four sections measure the impact of common muscle disease symptoms (weakness, locking (aka myotonia), pain and fatigue). Five sections measure the influence of the muscle disease on particular areas of life (activities, independence, relationships, emotions and body image). The last section is related to disease treatment. All responses are given in a seven-point Likert scale. The final score for each of nine sections is presented as a percentage of the maximum detrimental impact with a higher percentage indicating greater symptom impact or worse QoL. From the treatment section two scores are calculated (perceived treatment effect and expected treatment effect) as the trade-off between the positive and side effects of the treatment. Overall QoL score is calculated from five sections assessing the influence of the muscle disease on particular areas of life. In summary, INQoL includes 45 items, 10 sections, yielding 11 scores and one total score.
INQoL was cross-culturally adapted and translated using the standard recognized methodology [15]. INQoL was translated into Serbian by two translators, native Serbian speakers bilingual in English. The two independent translations were discussed by the translators and by experts in neuromuscular disorders (V.R.S. and S.P.) resulting in a first draft Serbian version of INQoL. This version was back translated into English by two translators, native English speakers bilingual in Serbian. These two translations were compared with original INQoL by the translators and the physicians (V.R.S. and S.P) resulting in the second draft Serbian version of INQoL. Second draft was tested in 8 patients with DM1 who were native speakers of Serbian. After minor corrections, athird and final Serbian version of the INQoL was agreed.
INQoL was validated using previously published guidelines [16]. Reliability analysis included internal consistency and reproducibility (test-retest reliability). We also assessed content-related validity, i.e. psychometric evaluation (item internal consistency and item discriminant validity), then construct-related validity (known-group validity), criterion-related validity (correlation between INQoL and MIRS) and concurrent validity (comparison between INQoL and SF-36 as well as comparison of MIRS with both of these QoL questionnaires).
Normality of data was tested by the Kolmogorov-Smirnov test. To compare the two patients groups, Mann-Whitney U test, Student’s t-test and χ2 test were used, as appropriate. Since INQoL is composed of 11 sections, internal consistency was analyzed for each section using Cronbach’s alpha (minimal standard was 0.70 for group comparisons and 0.90 for individual comparisons). Test-retest reliability was assessed by intraclass correlation coefficient (ICC) (minimal standard was 0.70 for group comparisons and 0.90 for individual comparisons). The mean, standard deviation and percentage of patients obtaining top score (ceiling effect) and bottom score (floor effect) were calculated. Item internal consistency was analyzed as Spearman’s correlation coefficient between an item in a domain and the domain score computed from all other items in the same domain (R ≥ 0.40 meaning appropriate inclusion of items within a specific domain). Internal convergence success was expressed as a percentage of the internal correlation coefficients above 0.40. Item discriminant validity was assessed as Spearman’s correlation coefficient between an item of a section and the other similar sections. Item discriminant validity failure was expressed as a percentage of correlations of items with own scales lower than correlations with other similar scales. Construct-related validity was assessed as an expected impact of gender (Mann-Whitney U test), age, education and duration of disease (Spearman’s correlation coefficient) on questionnaire scores. The relationship between MIRS and INQoL scores was analyzed by ANOVA representing criterion-related validity. The association between the INQoL scales and total score with each of the scales and SF-36 summary indexes was analyzed by Spearman’s correlation coefficient. In addition certain scales of both questionnaires were compared in terms of correlation with MIRS (linear regression analysis). In all analyses, significance testing was two-sided, with α sets at 0.05 for statistical significance and 0.01 for high statistical significance.
| Results | ▴Top |
Demographic and clinical features of investigated patients are listed in the Table 1.
 Click to view | Table 1. Demographic and Clinical Characteristics of Investigated DM1 Patients |
INQoL was self-completed in approximately 20 minutes. All patients found the Serbain INQoL questionnaire understandable and that the language used was appropriate and simple.
The internal consistency of the Serbian version of INQoL was excellent and satisfied the level for individual comparison in 7 of 10 scales (Cronbach’s alpha 0.903 - 0.961). Emotions and body image scales satisfied level for group comparison with Cronbach’s alpha of 0.898 and 0.864, respectively. However, internal consistency of the treatment section was somewhat below requested values (0.625) (Table 2).
 Click to view | Table 2. Reliability and Content-related Validity of INQoL in DM1 Patients |
A subgroup of 30 patients was tested for reproducibility. These patients did not differ from overall group (P > 0.05) except that they had better education (P < 0.05) (Table 1). Test-retest reliability of the questionnaire satisfied the level for individual comparison in 4 of 10 scales (ICC 0.910 - 0.979). Rest seven scales satisfied level for group comparison (ICC 0.713 - 0.895) (Table 2).
Mean scores of INQoL in DM1 patients are showed in the Table 3. Floor effect was significant for pain scale while ceiling effect was insignificant (Table 2).
 Click to view | Table 3. Scores on INQoL in DM1 Patients (n = 102) |
Item internal consistency was satisfactory except for treatment scales. For all scales internal convergence success was 100%, except treatment scores. Item discriminant validity was excellent with failure of 0 for each item (Table 3).
There was no difference in any INQoL score between male and female subjects (P> 0.05). Age of patients and duration of DM1 showed significant positive correlation with all INQoL scales (P < 0.01) except treatment scores (P > 0.05). Lower education was correlated with worse score on activities scale and with worse total QoL score (P < 0.05) (Table 4).
 Click to view | Table 4. Construct-related Validity of INQoL in DM1 Patients (n = 102) |
Positive correlation was observed between MIRS and all scales of INQoL (P < 0.01) except treatment domains (P > 0.05) (Fig. 1).
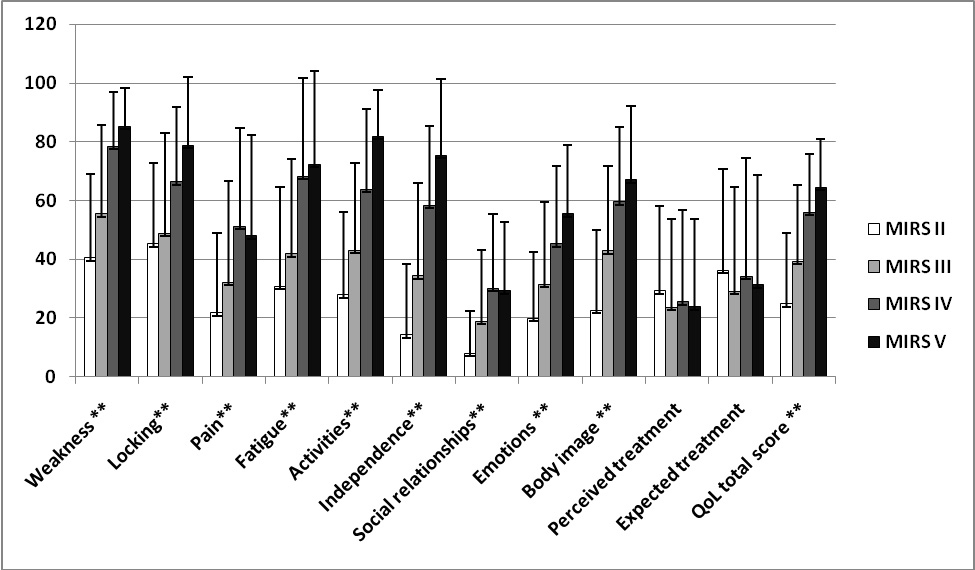 Click for large image | Figure 1. Criterion-related validity of INQoL in DM1 patients: correlation of INQoL scores with severity of disease (MIRS) (n = 102, **P < 0.01). |
There was a negative correlation between INQoL scales and SF-36 domains (as expected for the differing directions of their scoring) and the strongest correlations are listed in the Table 5. INQoL total score showed significant correlation with both PCS and MCS of SF-36 (P < 0.01). INQoL scales explained 43% of variance of MIRS, while comparable SF-36 domains explained 42% of variance of MIRS. PF score of SF-36 had better correlation with MIRS than comparable weakness and activities scales of INQoL. Pain scale of INQoL more significantly contributed to MIRS than comparable BP score of SF-36.
 Click to view | Table 5. Concurrent Validity of INQoL in DM1 Patients (n = 102) |
| Discussion | ▴Top |
Initial validations on INQoL included large but heterogeneous groups of patients with different muscular disorders [11, 12], while our study comprised only patients with DM1. Our data demonstrate that Serbian version of INQoL is understandable, easy to administer, reliable and valid QoL measure for use in DM1 and it is able to capture disease specific issues usually omitted by generic questionnaires.
The internal consistency of the Serbian version of INQoL was excellent except for treatment section. This may be explained by the fact that treatment section includes questions about positive and negative effects of perceived and expected treatment [11], thus intercorrelation between these items was expected to be low.
Reproducibility of INQoL was excellent which is in agreement with previous reports on a heterogeneous group of patients [12]. Of note, reproducibility was excellent for myotonia which is consistent with the clinician perception that myotonia in DM1 shows less fluctuation compared with that seen in myotonic dystrophy type 2 or non-dystrophyic myotonias [1].
In our patients the highest detrimental impact was found for weakness, locking, fatigue and activities and the lowest score was observed for pain, social relationships and emotions. Italian validation of the INQoL was performed on 1092 patients with different muscular disorders but examined criterion-validity in a subgroup 70 DM1 patients making it comparable to our group [12]. A similar profile of detrimental impact was observed in Italian DM1 subgroup and in our patients, but our patients scored worse in all INQoL scales in comparison to the Italian DM 1 patients [12]. This finding is consistent with our previous research [4] which showed that Serbian patients with DM1 scored worse also on SF-36 in comparison to Italian DM1 patients [5]. We speculated that relatively better health care, community services and overall economic situation in Italy in comparison to Serbia may improve patients’ quality of life since environmental factors are known to have a tremendous influence on lives of DM1 patients and their families [2, 17].
We found significant floor effect only for pain scale. This finding is not due to the weakness of the INQoL but it probably reflects the fact that pain is not present in 40% of DM1 patients [18, 19].
Item discriminant validity was excellent for all analyzed scales, while internal convergence success was excellent for all scales, except for treatment scores. This is due to the specific calculation of the treatment scores: they are expressed as the trade-off between the positive and side effects of the treatment [11].
We found that INQoL scores had significant positive correlation with age of patients, duration of disease and severity of muscular impairment, and they were only slightly affected by educational level and not related to patients’ gender. Previous researches on DM1 patients showed that age of patients [4, 5], disease duration and severity of muscular impairment [4, 5, 12] also similarly correlated with QoL. Weaker correlation was previously observed between educational level and QoL measured by SF-36 [4]. Some studies showed that female neuromuscular patients had worse QoL in comparison to male patients [12], but other studies did not find any gender differences in DM1 [4, 5].
INQol total index significantly correlated with SF-36 total score as well as with both PCS and MCS suggesting that INQoL captures both physical and mental limitations caused by muscular disorder. However, INQoL has the advantage of recording specific disease symptom impacts omitted by generic questionnaire such as locking and body image [11, 12]. Furthermore, our results suggested that INQoL better captures pain problems in DM1 patients than BP domain of the SF-36. INQoL also has the advantage that the effects of symptoms are separated from questions about life domains. This separation allows “shifts” in patients’ internal standards to be identified if satisfaction with life domains has altered independently from a change in perceived symptoms [11]. It may be possible that in DM1 in contrast to other disabling neuromuscular disorders adaptive coping behavior is reduced or even not present because of frontal lobe dysfunction and avoidant personality trait that have been previously reported in these patients [20, 21].
There are still several limitations to our study. The first one regards INQoL itself. INQoL has been so far validated on heterogeneous groups of muscle diseases having in common disability and chronic muscle impairment [11, 12] and it may be considered as a symptom-specific and not a disease-specific QoL questionnaire. However, our data confirmed its validity on a homogeneous group of patients with DM1. Second problem is that DM1 is a multi-system disease including, for example, cognitive impairment and respiratory complications [1, 22] that may not be captured by INQoL. Other specific QoL scales may be required for these aspects. Alternatively, further symptoms relevant to DM1 such as ptosis, swallowing difficulties, daytime sleepiness etc. [23] could be potentially added to the impact section of the INQoL but this would need additional validation. Finally, the cross-sectional design of our study precludes analysis of the responsiveness of INQoL. Since DM1 is a slowly progressive disease, the follow-up period for responsiveness analysis needs to be at least 5-10 years and this is going to be our next step.
Acknowledgments
We thank Dr. Michael Rose, who created INQoL, for helpful advice and comments he gave us during the final preparation of this paper.
This study was supported by the Ministry of Science of The Republic of Serbia (Grant No 175083).
Conflict of interests
Authors report no conflict of interests.
| References | ▴Top |
- Harper P. Myotonic dystrophy. 3rd ed. London: WB Saunders; 2001.
- Gagnon C, Chouinard MC, Laberge L, Veillette S, Begin P, Breton R, Jean S, et al. Health supervision and anticipatory guidance in adult myotonic dystrophy type 1. Neuromuscul Disord. 2010;20(12):847-851.
pubmed doi - Boyer F, Morrone I, Laffont I, Dizien O, Etienne JC, Novella JL. Health related quality of life in people with hereditary neuromuscular diseases: an investigation of test-retest agreement with comparison between two generic questionnaires, the Nottingham health profile and the short form-36 items. Neuromuscul Disord. 2006;16(2):99-106.
pubmed doi - Peric S, Rakocevic-Stojanovic V, Stevic Z, Basta I, Pavlovic S, Vujanac V, Marjanovic L, et al. Health-related quality of life in patients with myotonic dystrophy type 1 and amyotrophic lateral sclerosis. Acta Neurol Belg. 2010;110(1):71-77.
pubmed - Antonini G, Soscia F, Giubilei F, De Carolis A, Gragnani F, Morino S, Ruberto A, et al. Health-related quality of life in myotonic dystrophy type 1 and its relationship with cognitive and emotional functioning. J Rehabil Med. 2006;38(3):181-185.
pubmed doi - Kalkman JS, Schillings ML, van der Werf SP, Padberg GW, Zwarts MJ, van Engelen BG, Bleijenberg G. Experienced fatigue in facioscapulohumeral dystrophy, myotonic dystrophy, and HMSN-I. J Neurol Neurosurg Psychiatry. 2005;76(10):1406-1409.
pubmed doi - Laberge L, Dauvilliers Y, Begin P, Richer L, Jean S, Mathieu J. Fatigue and daytime sleepiness in patients with myotonic dystrophy type 1: to lump or split? Neuromuscul Disord. 2009;19(6):397-402.
pubmed doi - Tieleman AA, Jenks KM, Kalkman JS, Borm G, van Engelen BG. High disease impact of myotonic dystrophy type 2 on physical and mental functioning. J Neurol. 2011;258(10):1820-1826.
pubmed - MacDonald JR, Hill JD, Tarnopolsky MA. Modafinil reduces excessive somnolence and enhances mood in patients with myotonic dystrophy. Neurology. 2002;59(12):1876-1880.
pubmed - Penisson-Besnier I, Devillers M, Porcher R, Orlikowski D, Doppler V, Desnuelle C, Ferrer X, et al. Dehydroepiandrosterone for myotonic dystrophy type 1. Neurology. 2008;71(6):407-412.
pubmed doi - Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL). Neurology. 2007;68(13):1051-1057.
pubmed doi - Sansone VA, Panzeri M, Montanari M, Apolone G, Gandossini S, Rose MR, Politano L, et al. Italian validation of INQoL, a quality of life questionnaire for adults with muscle diseases. Eur J Neurol. 2010;17(9):1178-1187.
pubmed doi - Mathieu J, Boivin H, Meunier D, Gaudreault M, Begin P. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology. 2001;56(3):336-340.
pubmed - http://www.qualitymetric.com SF-36 Health survey (Original version) Language Recalls.
- Acquadro C, Conway K; Giroudet C, Mear I. Linguistic Validation Manual for Patient-Reported Outcomes (PRO) Instruments. Mapi Research Institute, 2004.
- Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11(3):193-205.
pubmed doi - Gagnon C, Noreau L, Moxley RT, Laberge L, Jean S, Richer L, Perron M, et al. Towards an integrative approach to the management of myotonic dystrophy type 1. J Neurol Neurosurg Psychiatry. 2007;78(8):800-806.
pubmed doi - Guy-Coichard C, Nguyen DT, Delorme T, Boureau F. Pain in hereditary neuromuscular disorders and myasthenia gravis: a national survey of frequency, characteristics, and impact. J Pain Symptom Manage. 2008;35(1):40-50.
pubmed doi - Jensen MP, Hoffman AJ, Stoelb BL, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil. 2008;89(2):320-328.
pubmed doi - Meola G, Sansone V, Perani D, Colleluori A, Cappa S, Cotelli M, Fazio F, et al. Reduced cerebral blood flow and impaired visual-spatial function in proximal myotonic myopathy. Neurology. 1999;53(5):1042-1050.
pubmed - Meola G, Sansone V, Perani D, Scarone S, Cappa S, Dragoni C, Cattaneo E, et al. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2). Neuromuscul Disord. 2003;13(10):813-821.
pubmed doi - Meola G, Sansone V. Cerebral involvement in myotonic dystrophies. Muscle Nerve. 2007;36(3):294-306.
pubmed doi - Romigi A, Izzi F, Pisani V, Placidi F, Pisani LR, Marciani MG, Corte F, et al. Sleep disorders in adult-onset myotonic dystrophy type 1: a controlled polysomnographic study. Eur J Neurol. 2011;18(9):1139-1145.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
