| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Short Communication
Volume 14, Number 1, June 2024, pages 37-42
Non-Invasive Neuromodulation for Episodic and Chronic Migraine Headache: Preliminary Findings on Feasibility of At-Home Transcranial Direct Current Stimulation With Remote Supervision
Alexander Mauskopa, b, Elizabeth Sengc, d, Jordan Van Zyle, Russell K. Portenoyc, e, f, Helena Knotkovac, e, f, g
aNew York Headache Center, New York, NY, USA
bDepartment of Neurology, SUNY Downstate Medical Center, Brooklyn, NY, USA
cDepartment of Neurology, Albert Einstein College of Medicine, The Bronx, NY, USA
dDepartment of Psychology, Yeshiva University, The Bronx, NY, USA
eMJHS Institute for Innovation in Palliative Care, New York, NY, USA
fDepartment of Family and Social Medicine, Albert Einstein College of Medicine, The Bronx, NY, USA
gCorresponding Author: Helena Knotkova, MJHS Institute for Innovation in Palliative Care, New York, NY 10006, USA
Manuscript submitted October 4, 2023, accepted November 17, 2023, published online January 10, 2024
Short title: Feasibility of At-Home tDCS for Headache
doi: https://doi.org/10.14740/jnr760
| Abstract | ▴Top |
Background: The outcomes of drug therapies in migraine vary and the development of novel non-pharmacological treatments is a priority. Non-invasive neuromodulation using transcranial direct current stimulation (tDCS) in small-sample studies with brief treatment protocols has shown preliminary efficacy in management of migraine symptoms. We have piloted a use of tDCS modified for applications of longer treatment protocols in home settings and conducted a pilot randomized sham-controlled study involving 60 daily at-home tDCS applications in migraine patients (targeted N = 60). The COVID-19 pandemic precipitated early study closure, and the final enrollment (N = 22) was insufficient to test efficacy. Here we report findings on treatment feasibility, adherence, and satisfaction.
Methods: Participants were enrolled from the New York metropolitan area in 2018 - 2020. Main eligibility criteria included diagnosis of episodic or chronic migraine, history of headache for ≥ 1 year and ≥ 4 days with headache per month during a 30-day baseline period. At-home tDCS with remote supervision delivered the current at intensity of 1.5 mA or sham for one 20-min session per day on 60 consecutive days. The feasibility was determined by the drop-out rate after treatment started. Adherence was measured as the proportion of days during the 60-day study period that the patient activated the device. Satisfaction was evaluated from the satisfaction survey completed after the 60-day use of the device.
Results: Thirty-six patients provided consent and were assessed for eligibility; 22 of them (17 F, 5 M, age of 38.4 ± 11.0 years) met eligibility criteria and were enrolled. Six patients dropped out after the intervention started; 16 patients (73% of enrolled) continued through the 60-day treatment. In this group, adherence was high; the mean (standard deviation (SD)) number of sessions per patient was 49.3 (13.1); the median was 52.5. All 16 patients were satisfied with education about tDCS and 13 (81%) found the use of the tDCS device easy. No significant adverse events occurred.
Conclusions: At-home tDCS with remote supervision is feasible in migraine patients. If efficacy is confirmed in future research, at-home tDCS could become a useful tool for patients with severe migraine headache.
Keywords: Chronic and episodic migraine; Non-invasive neuromodulation; Transcranial direct current stimulation; At-home tDCS with remote supervision; Symptom management
| Introduction | ▴Top |
Migraine is a highly prevalent disorder and is associated with high rates of disability and health care utilization [1-4]. In the USA, migraine affects approximately 17% of women and 6% of men, and 30% of those diagnosed report a frequency or severity of headache that justifies daily preventative therapy [5]. Abortive and preventative drug therapies are the mainstay therapy and numerous drugs are recommended [6]. Many nonpharmacological treatments - usually psychoeducational, cognitive, behavioral, or integrative - also are used [7] and the development of new approaches is a priority.
Non-invasive neuromodulation is a nonpharmacological approach with potential benefits for many types of chronic pain, including headache [8-14]. For the treatment of migraine, transcranial direct current stimulation (tDCS) via electrodes applied on the scalp is especially interesting given the growing evidence of efficacy in numerous conditions [15] and promising results from early trials in migraine populations [10, 15]. A recent meta-analysis of tDCS in migraine involving five randomized controlled trials with the total of 104 migraine patients found a significant reduction of pain intensity in active vs. sham tDCS treated patients and the effects persisted to the follow-up post-intervention period [9]. The method has a favorable side effect profile and minimal contraindications to its use [9-12], which further highlights its potential for adaptation into a relatively low-cost at-home therapy for long-term use [16].
An at-home tDCS device has been developed that incorporates technology to ensure safety, monitor adherence, and provide remote connection to support staff [16, 17]. We piloted the use of this device in a sample of patients with severe episodic or chronic migraine using a randomized design evaluating 60 days of daily at-home sessions. The study planned an assessment of feasibility, treatment adherence, patient satisfaction, and efficacy. The COVID-19 pandemic precipitated early study closure; however, and the final enrollment (N = 22) was insufficient to test efficacy (see clinicaltrials.gov NCT03874351). Treatment feasibility, adherence, and satisfaction could be assessed, however, and is reported herein.
| Materials and Methods | ▴Top |
The study was approved by the Ethical and Independent Review Services Institutional Review Board (IRB #19008-01) and conducted in compliance with all applicable ethical standards and guidelines. The study began in 2018 and was closed in 2020.
The design was double-blind, randomized, and sham-controlled. Patients were recruited from a headache specialty practice in the New York metropolitan area. Eligibility criteria included: 1) age 18 - 65 years, 2) diagnosis of episodic or chronic migraine, with or without aura, as defined by the International Classification of Headache Disorders third edition (ICHD-3), 3) history of headache for ≥ 1 year, 4) ≥ 4 days with headache per month, 5) no change in headache therapy during the preceding 3 months, and 6) able to follow instructions in English and provide consent. Patients receiving botulinum toxin injections, any calcitonin gene-related peptide (CGRP) monoclonal antibody, any N-methyl-D-aspartate (NMDA)-receptor antagonist, or any opioid or barbiturate drug for ≥ 2 days/week were excluded. The reason why these medications were excluded was that botulinum toxin injections and CGRP monoclonal antibody treatments may provide incremental relief with repeated administration and those who frequently use opioid and barbiturate drugs tend to have rebound headaches that do not respond well to any therapy. NMDA blockers were excluded because tDCS effect in part depends on NMDA plasticity. Other exclusions included history of seizures, severe head trauma, brain surgery, implants in the head or neck, skin disorders at or near the tDCS electrode locations, any unstable medical or psychiatric disorder, and the use of any investigational treatment within the past 30 days.
The study period included baseline data collection (30 days) and study intervention (60 days). On each day of the study period, patients completed a headache and medication diary. After the baseline period, eligible patients were randomized in a double-blind manner to either active tDCS or sham stimulation.
The tDCS device (Soterix mini-CT, Soterix Medical Inc., New York, NY) was programmed by the manufacturer to deliver active tDCS at a current intensity of 1.5 mA or sham treatment for one 20-min session per day. The equipment included a size-fitted headband, saline presoaked electrodes (5 × 5 cm) clipped into the headband, a battery-powered device, and a tablet allowing videoconferencing for quality checks and remote support as needed. The headband was constructed so that a trained patient could position it consistently across treatment sessions, with the anode over the area of C4 (the left primary motor cortex) of the 10-20 electroencephalography (EEG) system and the cathode over the contralateral supraorbital region [18].
The randomization was done using a computer-generated randomization list prepared by the study statistician using the block-of-six method. Both the patient and study personnel involved in the study development, study procedures and data collection except for statistician were blinded to the sham vs. active tDCS treatment assignment. tDCS devices were programmed by the manufacturer to sham or active tDCS mode in accordance with the randomization list. After randomization, patients were trained in the use of the tDCS device at home and performed the first study session (active/sham) with in-person supervision. Thereafter, patients self-administered one session per day for 60 days and could access study personnel via videoconference whenever needed. Safety monitoring was performed during the 60 days of study intervention, and 30 days thereafter, via videoconference or telephone. A satisfaction survey, administered when the device was returned after 60 days, included seven statements related to tDCS training and use; respondents indicated if they strongly agree, agree, neither agree nor disagree, disagree, or strongly disagree with the statements.
Descriptive statistics were used to evaluate feasibility, adherence, and satisfaction. The feasibility of the at-home tDCS was determined by the proportion of eligible patients who could be trained and the drop-out rate after treatment started. Treatment adherence was measured as the proportion of days during the 60-day study period that the patient activated the device. Satisfaction was measured using the satisfaction survey completed at visit 3.
| Results | ▴Top |
Thirty-six patients provided consent and were assessed for eligibility; 22 met eligibility criteria and were enrolled (Table 1). The 17 women and five men had an average age of 38.4 (standard deviation (SD): 11.0) years. Most were White (15/22; 68.2%) and single (14/22; 63.6%) and had completed college (16/21; 72.7%). All patients had episodic migraine and 15 met criteria for chronic migraine.
 Click to view | Table 1. Baseline Characteristics (N = 22) |
All eligible patients who were offered the tDCS trial agreed to proceed after learning about the device. Five patients (23% of those who were enrolled, including three receiving active tDCS and two receiving sham treatment) dropped out after starting daily sessions because they perceived the approach as time-consuming.
One patient was unable to follow study procedures and was discontinued by the investigators. The remaining 16 patients (73% of those enrolled; seven receiving active tDCS and nine receiving sham) continued through the 60-day treatment period. In this group, adherence to the daily treatment schedule was high, as measured by the proportion of 60 daily sessions that were performed by each patient (Table 2). The mean (SD) number of sessions per patient was 49.3 (13.1); the median was 52.5 (range 13 - 60). Comparing the initial 30 days of the study period with the subsequent 30 days revealed no difference in adherence. Eleven patients (68.8%) completed at least 80% of the daily treatments, and four patients (25%) completed all 60 study treatments.
 Click to view | Table 2. Adherence to the Prescribed 60-Day Intervention (N = 16) |
The adverse events were minor. One participant in the active tDCS group had three instances of nausea and one participant in the sham group reported two occasions of sore throat.
On the satisfaction survey, all 16 patients “agreed/strongly agreed” with the statement “I was satisfied with the education and information I received before using the device”, 14 agreed/strongly agreed with the statement “I was comfortable using the tDCS device”, and 13 agreed/strongly agreed with the statement “I find the use of tDCS device easy” (Fig. 1). Twelve participants “agreed/strongly agreed” with the statement “If I were offered this device and equipment in the future, I would use it again” and “With the help of the tDCS device I am more confident managing the symptoms at home”. Eleven would recommend the device to others, and 10 agreed/strongly agreed that they were “satisfied with the overall experience of using the tDCS device”.
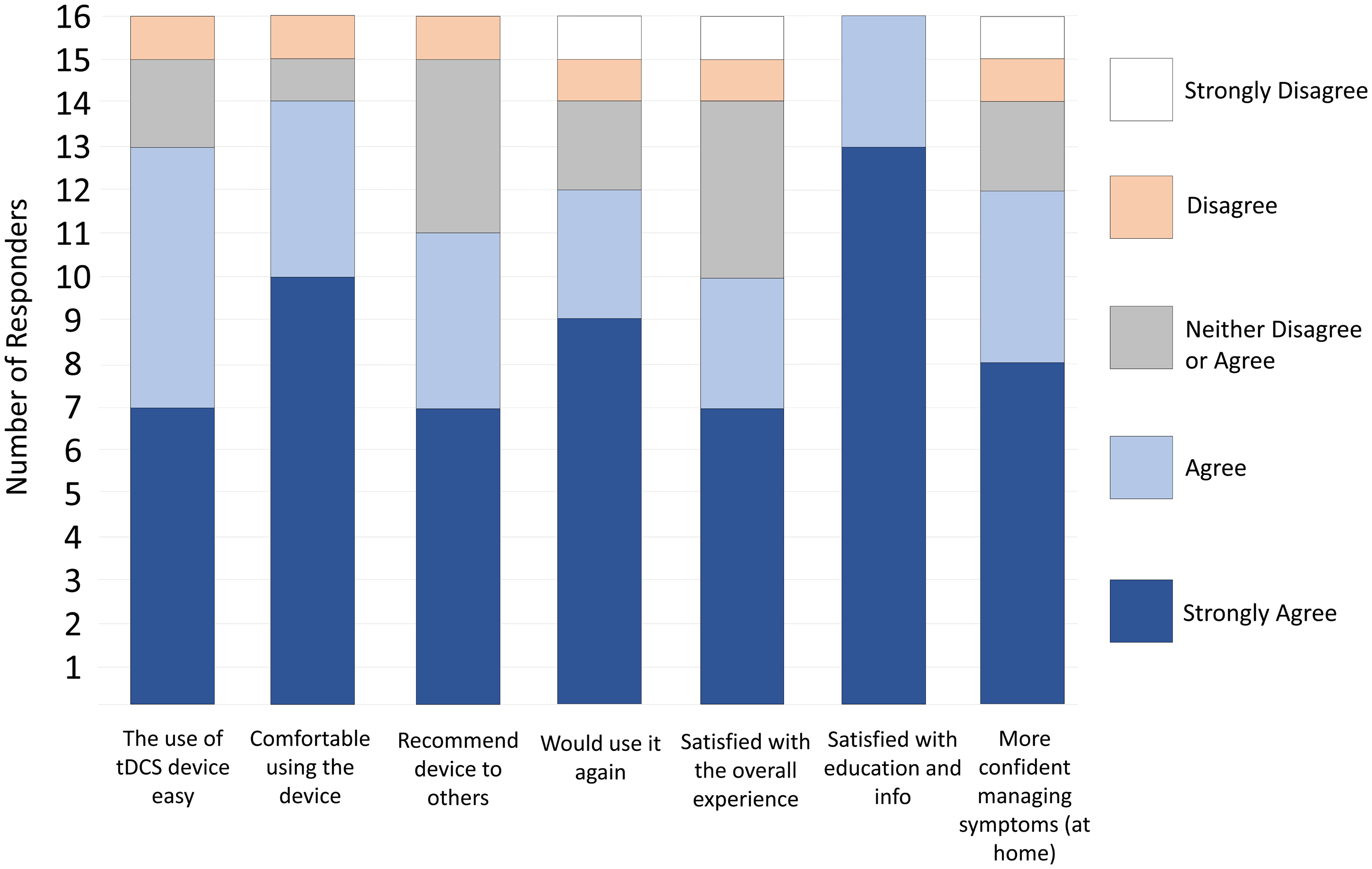 Click for large image | Figure 1. Satisfaction with the device and procedure (n = 16). |
| Discussion | ▴Top |
This trial was undertaken in a population with severe migraine headache to evaluate the feasibility of treatment with an at-home tDCS device, patient adherence to a 60-day treatment period, and satisfaction with the approach, and to obtain initial information about treatment efficacy. The onset of the COVID-19 pandemic interrupted the study; however, and it was terminated early. The sample size accrued prior to closure was insufficient to evaluate efficacy but adequate to assess other endpoints. Feasibility was supported by the observation that almost three-quarters of those who consented and were eligible could be trained in the use of the tDCS device and initiate daily treatment. Both adherence to a daily stimulation protocol and patient satisfaction after 60 days of daily treatment were, respectively, very high. There were no significant adverse events among those who received active treatment. The findings were in accordance with the anticipation that the at-home remotely-supervised intervention would be feasible and well accepted by migraine patients.
Although the main hypothesis that the 2-month neuromodulation protocol would significantly decrease the number of migraine days and decrease migraine pain and other symptoms could not be tested, studies of neuromodulation therapies - both tDCS and transcranial magnetic stimulation (TMS) - have addressed the therapeutic potential. Although this work is limited [15], the data support the potential utility of both tDCS and TMS for migraine headache [9-14]. TMS is feasible and acceptable [10], and is now available through a hand-held, patient-administered device for prevention and acute treatment in migraine with aura. This type of device, like the at-home tDCS therapy evaluated in this study may be particularly useful for chronic conditions, such as migraine, because they facilitate longer stimulation protocols. At-home tDCS has low patient burden and is relatively low cost and can be adapted for both stand-alone and add-on treatment protocols. Our findings support prior studies that suggest the acceptability of this at-home tDCS device with remote supervision [19-21] and did not observe the type of patient difficulties (including a drop-out rate of 41%) that have occurred when remote supervision was not available [22]. Remote supervision also may reduce variation in the use of the device and increase safety monitoring.
The main limitation of our study is the low sample size. Not only the main hypothesis on tDCS efficacy could not be tested, but the insufficient sample size also limited exploratory analysis. From that perspective, some clinically relevant questions, such as the role of specific demographic characteristics (e.g., sex, ethnicity) as modulators of tDCS adherence, could not be examined and our findings on adherence, feasibility and satisfaction are preliminary. Regardless of the limitations, we hope that our study can facilitate further research in the field of home-based tDCS. From the perspective of clinical utility of tDCS, evidence gaps remain in multiple aspects, including neurophysiological mechanisms and modulators of tDCS effects, dose optimization and the development of patient-tailored protocols.
Overall, our findings suggest that an at-home tDCS device with remote supervision, implemented with a training session and a device that provides replicable headband placement and a time-lock that limits treatment to once daily, could be used in the management of migraine headache. If efficacy is confirmed in future research, at-home tDCS could become a useful non-invasive and non-pharmacological therapy for patients with migraine headache.
Acknowledgments
The study was supported by a grant from the Price Farbman Foundation.
Financial Disclosure
The study was supported by a grant from the Price Farbman Foundation.
Conflict of Interest
None to declare.
Informed Consent
Patients provided signed informed consent.
Author Contributions
HK, RKP and AM drafted the manuscript. ES and JV contributed to data evaluation and created the tables and figures. All authors approved the final version of this manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Amiri P, Kazeminasab S, Nejadghaderi SA, Mohammadinasab R, Pourfathi H, Araj-Khodaei M, Sullman MJM, et al. Migraine: a review on its history, global epidemiology, risk factors, and comorbidities. Front Neurol. 2021;12:800605.
doi pubmed pmc - Buse DC, Fanning KM, Reed ML, Murray S, Dumas PK, Adams AM, Lipton RB. Life with migraine: effects on relationships, career, and finances from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache. 2019;59(8):1286-1299.
doi pubmed pmc - Safiri S, Pourfathi H, Eagan A, Mansournia MA, Khodayari MT, Sullman MJM, Kaufman J, et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain. 2022;163(2):e293-e309.
doi pubmed - Lipton RB, Nicholson RA, Reed ML, Araujo AB, Jaffe DH, Faries DE, Buse DC, et al. Diagnosis, consultation, treatment, and impact of migraine in the US: Results of the OVERCOME (US) study. Headache. 2022;62(2):122-140.
doi pubmed pmc - Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343-349.
doi pubmed - Tfelt-Hansen PC. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2013;80(9):869-870.
doi pubmed - Puledda F, Shields K. Non-pharmacological approaches for migraine. Neurotherapeutics. 2018;15(2):336-345.
doi pubmed pmc - Zheng Y, Liu CW, Hui Chan DX, Kai Ong DW, Xin Ker JR, Ng WH, Wan KR. Neurostimulation for chronic pain: a systematic review of high-quality randomized controlled trials with long-term follow-up. Neuromodulation. 2023;26(7):1276-1294.
doi pubmed - Cai G, Xia Z, Charvet L, Xiao F, Datta A, Androulakis XM. A systematic review and meta-analysis on the efficacy of repeated transcranial direct current stimulation for migraine. J Pain Res. 2021;14:1171-1183.
doi pubmed pmc - Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache. 2019;59(3):339-357.
doi pubmed - Feng Y, Zhang B, Zhang J, Yin Y. Effects of non-invasive brain stimulation on headache intensity and frequency of headache attacks in patients with migraine: a systematic review and meta-analysis. Headache. 2019;59(9):1436-1447.
doi pubmed - Zhong J, Lan W, Feng Y, Yu L, Xiao R, Shen Y, Zou Z, et al. Efficacy of repetitive transcranial magnetic stimulation on chronic migraine: A meta-analysis. Front Neurol. 2022;13:1050090.
doi pubmed pmc - Ahdab R, Mansour AG, Khazen G, El-Khoury C, Sabbouh TM, Salem M, Yamak W, et al. Cathodal transcranial direct current stimulation of the occipital cortex in episodic migraine: a randomized sham-controlled crossover study. J Clin Med. 2019;9(1):60.
doi pubmed pmc - Mansour AG, Ahdab R, Khazen G, El-Khoury C, Sabbouh TM, Salem M, Yamak W, et al. Transcranial direct current stimulation of the occipital cortex in medication overuse headache: a pilot randomized controlled cross-over study. J Clin Med. 2020;9(4):1075.
doi pubmed pmc - Shirahige L, Melo L, Nogueira F, Rocha S, Monte-Silva K. Efficacy of noninvasive brain stimulation on pain control in migraine patients: a systematic review and meta-analysis. Headache. 2016;56(10):1565-1596.
doi pubmed - Charvet LE, Shaw MT, Bikson M, Woods AJ, Knotkova H. Supervised transcranial direct current stimulation (tDCS) at home: A guide for clinical research and practice. Brain Stimul. 2020;13(3):686-693.
doi pubmed - Riggs A, Patel V, Paneri B, Portenoy RK, Bikson M, Knotkova H. At-home transcranial direct current stimulation (tDCS) with telehealth support for symptom control in chronically-ill patients with multiple symptoms. Front Behav Neurosci. 2018;12:93.
doi pubmed pmc - Knotkova H, Riggs A, Berisha D, Borges H, Bernstein H, Patel V, Truong DQ, et al. Automatic M1-SO montage headgear for transcranial direct current stimulation (TDCS) suitable for home and high-throughput in-clinic applications. Neuromodulation. 2019;22(8):904-910.
doi pubmed - Dobbs B, Pawlak N, Biagioni M, Agarwal S, Shaw M, Pilloni G, Bikson M, et al. Generalizing remotely supervised transcranial direct current stimulation (tDCS): feasibility and benefit in Parkinson's disease. J Neuroeng Rehabil. 2018;15(1):114.
doi pubmed pmc - Pilloni G, Vogel-Eyny A, Lustberg M, Best P, Malik M, Walton-Masters L, George A, et al. Tolerability and feasibility of at-home remotely supervised transcranial direct current stimulation (RS-tDCS): Single-center evidence from 6,779 sessions. Brain Stimul. 2022;15(3):707-716.
doi pubmed - Kasschau M, Reisner J, Sherman K, Bikson M, Datta A, Charvet LE. Transcranial direct current stimulation is feasible for remotely supervised home delivery in multiple sclerosis. Neuromodulation. 2016;19(8):824-831.
doi pubmed - Hagenacker T, Bude V, Naegel S, Holle D, Katsarava Z, Diener HC, Obermann M. Patient-conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. J Headache Pain. 2014;15(1):78.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
