| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Original Article
Volume 14, Number 2, August 2024, pages 68-73
Prevalence, Clinical Profile and Influence of Onset-to-Treatment Time for Subarachnoid Hemorrhage on Quality of Life in Patients: A Retrospective Study of a Decade
Guilherme dos Reis Guimaraesa, Joao Pedro Mota Limaa, Caio Bolfer da Silvaa, Enrico Garcia Panuccia, Rodrigo Ferrari Fernandes Naufalb, Adib Saraty Malveirac, Lorenna Izadora Capovilla Martins Gonzalez-Reyesc, Marcos Natal Rufinod, e, f, Margarete Jardinetti de Oliveirad
aFaculty of Medicine, University of West Paulista, Presidente Prudente, Sao Paulo, Brazil
bDepartment of Medical Residency in Neurology, Presidente Prudente Regional Hospital, Sao Paulo, Brazil
cNeurology Department, Presidente Prudente Regional Hospital, Sao Paulo, Brazil
dPhysiology Department, Faculty of Medicine, University of West Paulista, Presidente Prudente, Sao Paulo, Brazil
eHealth Technology Assessment Nucleus, Faculty of Medicine, University of West Paulista, Presidente Prudente, Sao Paulo, Brazil
fCorresponding Author: Marcos Natal Rufino, Physiology Department, Faculty of Medicine, University of West Paulista, Presidente Prudente, Sao Paulo, Brazil
Manuscript submitted December 8, 2023, accepted July 25, 2024, published online August 10, 2024
Short title: Influence of OTT for SAH on Quality of Life
doi: https://doi.org/10.14740/jnr769
| Abstract | ▴Top |
Background: The objective of the current study was to outline the clinical profile of patients diagnosed with subarachnoid hemorrhage (SAH) due to intracranial aneurysm rupture, treated at a reference hospital in the interior of Sao Paulo, and to investigate the time elapsed between the ictus and the surgical approach and its relationship with motor deficits evaluated according to the modified Rankin Scale (MRS).
Methods: This is a retrospective cohort, with data collection and analysis of patients with SAH between 2010 and 2020.
Results: The results showed no correlation between MRS and: sex (P = 0.3459 nonparametric Mann-Whitney test), age range, and ethnicity (P = 0.5451 and P = 0.513, respectively, nonparametric Kruskal-Wallis test), affected arteries (P = 0.4801 nonparametric test of Kruskal-Wallis), and onset-to-treatment time for hemorrhage (rho = 0.02; P = 0.8204 in Spearman’s nonparametric correlation test), adopting 5% significance.
Conclusions: The prevalence of SAH in the hospital studied, and the profile and clinical aspects of the patients treated are similar to existing data in the literature. The onset-to-treatment time and site of the aneurysm did not influence the prognosis of the patients, who presented slightly better MRS levels than those found in the literature.
Keywords: Subarachnoid hemorrhage; Encephalic vascular accident; Intracranial aneurysm; Prevalence
| Introduction | ▴Top |
Subarachnoid hemorrhage (SAH) is a neurological emergency that requires adequate diagnosis and treatment to prevent rebleeding, reduce the risk to life, and improve the patient’s prognosis [1]. More than 80% of non-traumatic SAH are due to intracranial aneurysms [2]. The incidence of SAH from ruptured intracranial aneurysms varies between six and 10 cases per 100,000 person-years in the USA [3], and approximately 30% to 40% of affected patients die. Among those who survive, three out of five patients have sequelae that cause some degree of disability [4]. This weakness can be classified according to the modified Rankin Scale (MRS) [5, 6]. Despite advances, the wide range of levels of severity and the complex evolution of the disease pose challenges for therapeutic efficacy [1].
The treatment of SAH involves surgical approaches - craniotomy to clip the aneurysm or endovascular procedures. Both approaches aim to prevent aneurysm rebleeding, the earlier complication that increases mortality and morbidity [7, 8]. In addition to the technique employed, the ideal moment for intervention after aneurysmal SAH is a matter of discussion and controversy; since, despite the evolution of approaches, rebleeding, cerebral hydrocephalus, and vasospasm are major causes of morbidity and mortality in previously treated patients and represent common and significant complications [9-11]. Persistent post-SAH symptoms such as headache, neurocognitive sequelae, depression, and anxiety are poorly understood, despite the important harm to the patient. Early management to prevent these sequelae should be considered as important as the others, respecting the individuality of each case [12].
The global burden of cerebrovascular accident (CVA) due to SAH remains enormous, and the generalized use of guidelines elaborated from populations of relatively homogeneous ethnic, racial, and socioeconomic levels imposes limitations regarding their use in communities with few resources or with underrepresented groups [13]. Therefore, there is an ongoing need to understand disease trends and their impact in each country, to guide policy decisions and health planning [14]. The objective of the current study was to outline the clinical profile of patients diagnosed with acute SAH due to intracranial aneurysm rupture, treated at a reference hospital in the interior of the state of Sao Paulo, Brazil, and to investigate the time elapsed between the ictus and the surgical approach and its relationship with motor deficit evaluated according to the MRS.
| Materials and Methods | ▴Top |
Our study was approved by the Ethics and Research Committee of the Presidente Prudente Regional Hospital and the Ethics Committee of the University of West Paulista, and therefore we were authorized to access the medical records, and the informed consent form was waived by the appropriate committees. This study was conducted by the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [15]. The research protocol, which included efforts to ensure anonymity and minimize the risks for patients whose medical records were collected, is registered and available on “Plataforma Brasil” [16] (CAAE: 52326421.0.0000.5515).
Study design, location, and date of data collection
This is an observational, retrospective cohort study, with the collection and analysis of data recorded in the medical records (physical and/or electronic) of patients at the Hospital Regional de Presidente Prudente (HRPP), in the period between 2010 and 2020. Data collection was carried out between November 2021 and March 2022.
Included participants and exclusion criteria
All medical records of patients affected by SAH, treated at the HRPP during the period covered by the study, who underwent surgical treatment to control SAH and with clear postoperative evolution data were included. Records with incomplete data or that did not meet the objectives of this study were excluded.
Parameters of interest
To profile the patients and correlate the onset-to-treatment time for SAH after rupture of the intracranial aneurysm with possible motor deficits, we collected patient data (age at the time of SAH, sex, race) and the time elapsed between rupture of the aneurysm and the surgical intervention. Brain regions and affected arteries were investigated to assess the influence of aneurysm location on motor deficit. The result of the patient’s clinical/motor assessment at the time of hospital discharge was collected for classification of the patient in the MRS.
Patients were divided into seven different levels according to the MRS, which classifies the motor deficit into levels ranging from zero (no symptoms) to six (death) [17].
Statistical analysis
Preliminary data were submitted to the Shapiro-Wilk normality test. The nonparametric Kruskal-Wallis test was performed to compare patient MRS scores versus SAH arteries or sites and MRS scores versus patient ethnicity. The analyses to compare the influence of sex were performed with the nonparametric Mann-Whitney test. To correlate the MRS scores with the onset-to-treatment time variable, Spearman’s nonparametric correlation test was used. All analyses were conducted in the R program, adopting a 5% significance level [18].
| Results | ▴Top |
SAH prevalence and patient profile
The study evaluated 266 medical records of patients diagnosed with acute SAH during the years 2010 to 2020. The data indicated a prevalence of 0.13%. Among 266 patients with the condition, 180 medical records did not meet the inclusion criteria and were discarded from the study, as they contained insufficient data on clinical follow-up. The 86 medical records that met the inclusion criteria were analyzed, and information was collected on sex, ethnicity, affected artery, time elapsed until surgical intervention, and follow-up with physical examination and clinical evaluation, which allowed the classification of the patient in the MRS. The profile of the patients is shown in Table 1.
 Click to view | Table 1. Patient Profile |
The analyses showed that most of the patients affected by SAH in the period studied were women. The mean age of the patients was 62 years (± 11), with over 60% being in their fifth or sixth decade of life.
Clinical aspects: onset-to-treatment time, location of SAH, and patient’s MRS score
To facilitate the analyses, patients were grouped into three groups, according to the onset-to-treatment time: 1) group 1: onset-to-treatment time between 0 and 72 h; 2) group 2: onset-to-treatment time between 72 and 192 h; 3) group 3: onset-to-treatment time above 192 h after the rupture of the aneurysm (Fig. 1).
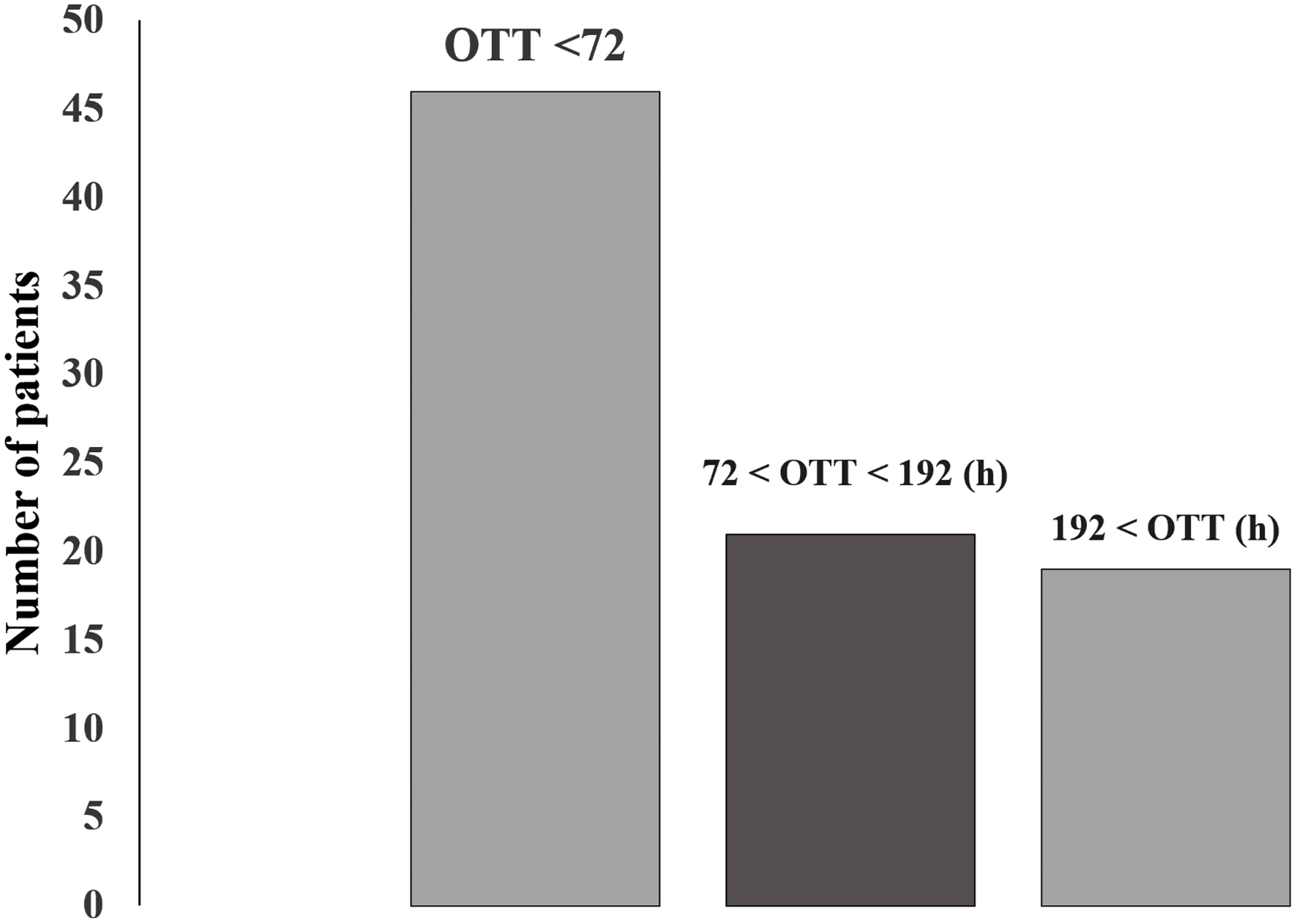 Click for large image | Figure 1. Distribution of patients according to onset-to-treatment time (OTT). |
Onset-to-treatment time is the time between ictus and surgical approach, ranging from a few hours (< 24 h) to 26 days. Most patients (52%) were treated within 72 h after the ictus; however, the mean time to start treatment was 144 h (6 days) after the ictus.
Of the 86 patients, eight patients (9%) had two simultaneous aneurysms, totaling 94 aneurysms. Of the total number of aneurysms, 93.61% occurred in the anterior circulation of the brain. The compromised arteries are shown in Table 2.
 Click to view | Table 2. Site of the SAH |
Table 3 shows that, according to the outcome MRS, most patients (approximately 68.5%) presented no sequelae or presented sequelae that did not cause significant motor impairment. In contrast, approximately 20.5% of patients developed severe sequelae or died.
 Click to view | Table 3. Outcome Classification of Patients According to the Modified Rankin Scale |
Statistical analyses were carried out to investigate the influence of patient characteristics (gender, age, ethnicity, and location of the aneurysm) on the prognosis, and they were correlated with the respective patient’s score on the MRS. The results of the analyses showed no significant correlation between the scores according to the MRS and the groups: sex (P = 0.3459 in the nonparametric Mann-Whitney test), age range, and ethnicity (P = 0.5451 and P = 0.513, respectively in the test of nonparametric Kruskal-Wallis). There was also no correlation between affected arteries/sites and MRS scores (P = 0.4801 in the nonparametric Kruskal-Wallis test). Statistical analyses showed no significant correlation between MRS and onset-to-treatment time (rho = 0.02, P = 0.8204 in Spearman’s nonparametric correlation test), adopting a 5% significance level.
| Discussion | ▴Top |
To collect and share data on the prevalence and clinical profile of the patient that may contribute to increases in the appropriate management, in addition to relating the onset-to-treatment time and the site of hemorrhage to the level of motor impairment of the patient in the post-SAH period, we carried out a retrospective study and collected data accumulated over a decade in the medical files of a referral hospital for highly complex procedures in the western region of the interior of the state of Sao Paulo. The characteristics of this study design are advantageous, as they allow the observation of various aspects related to the patient, the clinic, and even the prognosis, elucidating the temporal relationship between exposure and disease [19].
Among patients admitted to the hospital, a prevalence of 0.13% was detected, or one patient with SAH for every 1,000 hospitalized. Worldwide, pre-hospital and in-hospital mortality rates remain high, and the increased incidence of SAH in the older population corroborates the reasoning that these numbers do not reflect the prevalence of SAH in the general regional population, given that pre-hospital mortality is high for this condition, ranging from 20% to 26% [13, 20].
The data collected in the current study indicate that white women aged close to 61 years old are more prone to SAH, and these data are consistent with those found in the literature [21-23]. In fact, recent studies report a higher relative risk (RR) for the incidence of SAH in women (RR = 1.3), when compared to men, especially those aged over 55 years [24, 25]. Although the exact explanation for the higher risk in women in this age group has not been established, this fact coincides with the beginning of menopause, when there is a decrease in hormone production and a greater propensity for vascular diseases, associated with the smaller caliber and, therefore, more fragile vessels in women [26]. These vessels, with advancing age, lose elasticity and become more rigid, predisposing to hypertension. In the Brazilian population, studies have pointed to an association between systemic arterial hypertension and sex. In particular, in the state of Sao Paulo, hypertension is present in a greater number of women in this age group compared to men [27]. These peculiarities related to middle-aged women may be linked to the pathophysiological mechanisms responsible for the appearance of intracranial aneurysms, which are still debated. It is believed that the smaller amount of connective tissue surrounding the cerebral vessels [28], attenuated tunica media, and lack of external elastic lamina of intracranial arteries may be important etiological features. In addition to these factors, arterial hypertension coexists in a high percentage of patients who develop SAH and has been proposed as an etiological factor, although the exact relationship is not completely defined [29, 30].
Analyses of the data extracted from the medical records showed that the time elapsed between the onset of hemorrhage and the surgical intervention, and the affected artery were not decisive factors for the patient’s prognosis according to the MRS. These data are similar to those found in the literature [22, 23, 29]. The MRS is the most prevalent functional outcome measure in contemporary research, aiming to classify the disability presented by the patient, however, classification of the level of the patient in the MRS requires a clinical examination, which gives the measure moderate reliability, since it depends on the doctor’s skills [5]. A recent study pointed to the need to use consolidated scales for clinical evaluation before and during post-SAH recovery treatment. These scales can facilitate the identification of associated complications and optimize patient outcomes [13].
Despite the data obtained in this research and presented here, the updated guidelines for the management of patients with aneurysmal SAH - “Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association” [13] - recommend intervention, either by endovascular coiling or neurosurgical clipping, to prevent rebleeding and reduce lethality. However, the guidelines also recognize that despite the advances, there is increasing evidence of lasting morbidities related to cognition and/or reduced quality of life due to damage to cognitive and motor areas respectively [31].
The average time between the ictus and the surgical intervention of the patients studied in this research was 144 h (6 days), however, the majority were operated on 72 h. Despite this, our statistical analyses did not point to significant differences in the flow of the patients according to the MRS, regardless of the time elapsed until the surgical procedure. The disagreement between the data found here and the literature is likely justified by the low number of cases analyzed [32]. Previous studies agree that early treatment of SAH (between 0 and 3 days) reduces the risk of rebleeding and reduces mortality and patient dependence; the authors consider that the benefits for the patient are inversely proportional to the time elapsed for the surgical intervention [11, 33-35]. Another study suggested that the choice of surgery modality may vary, according to the profile, site of the aneurysm, and clinical conditions of the patient [13].
The measurement of the prognosis for the patients included in our investigation pointed out that the largest portion (44%) did not have sequelae, either motor or cognitive, and less than one-fifth (18%) died. These findings demonstrate an improved prognosis for affected patients compared to previous studies [23]. We emphasize that the data presented were extracted from the medical records of patients treated at a reference hospital for highly complex procedures, and this fact could explain the better prognosis of the patient. Studies indicate that hospital resources and the volume of cases and experience of the medical staff involved in treating SAH cases are related to the patient’s prognosis. Decreased mortality rates have been demonstrated when patients are managed by experienced cerebrovascular surgeons and neuroendovascular interventionalists in hospitals with higher case volumes and when care is provided in specialized neurological care units [13].
Finally, it is necessary to consider that there may be questionable data accuracy, or even incomplete medical records (which were excluded). This situation reflects the retroactive nature of the study, implying the low sample size, which in turn reduces the statistical sensitivity. However, the data presented here contribute to alerting medical professionals about the prevalence of SAH, the risk factors involved, the importance of choosing the appropriate treatment, and the challenges to provide a favorable prognosis for the patient. Likewise, studies aimed at elucidating the determinants of prognosis and validation of standardized scales for the assessment of SAH are necessary.
Conclusions
The prevalence of SAH in the studied hospital, and the profile and clinical aspects of the patients treated are similar to existing data in the literature. The onset-to-treatment time and site of the aneurysm did not influence the prognosis of the patients, who had slightly better MRS scores than those found in the literature.
Acknowledgments
For this research of such relevance to medical practice, we could not fail to thank our esteemed professors and key figures who guided the scientific production and were always willing to cooperate. Therefore, our gratitude goes to Doctors Margarete Jardinetti de Oliveira, Marcos Natal Rufino, and Rodrigo Ferrari Fernandes Naufal. We would also like to thank all the collaborators at the Presidente Prudente Regional Hospital, a true temple of medical education and research, for providing excellent service to the local population.
Financial Disclosure
There was no funding or financial incentive for the study, which was conducted using the researcher’s financial resources.
Conflict of Interest
The authors declare that there are no conflicts of interest of a personal, commercial, academic, political and or financial nature, in the process of reviewing and publishing the article above.
Informed Consent
Our study was approved by the Ethics and Research Committee of the Presidente Prudente Regional Hospital and the Ethics Committee of the University of West Paulista, and therefore we were authorized to access the medical records, and the informed consent form was waived by the appropriate committees.
Author Contributions
Guilherme dos Reis Guimaraes: study design, literacy search, writing and editing of the manuscript, data collection, and translation. Joao Pedro Mota Lima: literacy search, writing of the manuscript, and data collection. Caio Bolfer da Silva: data collection, references, graphics, and tables. Enrico Garcia Panucci: data collection, references, editing of the manuscript. Rodrigo Ferrari Fernandes Naufal: literacy search. Adib Saraty Malveira: literacy search. Lorenna Izadora Capovilla Martins Gonzalez-Reyes: literacy search. Marcos Natal Rufino: editing of the manuscript, and translation. Margarete Jardinetti de Oliveira: study design, and literacy search.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Chung DY, Abdalkader M, Nguyen TN. Aneurysmal subarachnoid hemorrhage. Neurol Clin. 2021;39(2):419-442.
doi pubmed pmc - Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44(12):3613-3622.
doi pubmed - Ajiboye N, Chalouhi N, Starke RM, Zanaty M, Bell R. Unruptured cerebral aneurysms: evaluation and management. ScientificWorldJournal. 2015;2015:954954.
doi pubmed pmc - Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635-642.
doi pubmed - Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke. 2009;40(10):3393-3395.
doi pubmed - Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):S6-S16.
doi pubmed - Wilkinson DA, Heung M, Deol A, Chaudhary N, Gemmete JJ, Thompson BG, Pandey AS. Cerebral aneurysms in autosomal dominant polycystic kidney disease: a comparison of management approaches. Neurosurgery. 2019;84(6):E352-E361.
doi pubmed pmc - Connolly ES, Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711-1737.
doi pubmed - Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4(4):432-446.
doi pubmed pmc - Tykocki T, Czyz M, Machaj M, Szydlarska D, Kostkiewicz B. Comparison of the timing of intervention and treatment modality of poor-grade aneurysmal subarachnoid hemorrhage. Br J Neurosurg. 2017;31(4):430-433.
doi pubmed - Dorhout Mees SM, Molyneux AJ, Kerr RS, Algra A, Rinkel GJ. Timing of aneurysm treatment after subarachnoid hemorrhage: relationship with delayed cerebral ischemia and poor outcome. Stroke. 2012;43(8):2126-2129.
doi pubmed - Sorrentino ZA, Laurent D, Hernandez J, Davidson C, Small C, Dodd W, Lucke-Wold B. Headache persisting after aneurysmal subarachnoid hemorrhage: a narrative review of pathophysiology and therapeutic strategies. Headache. 2022;62(9):1120-1132.
doi pubmed - Hoh BL, Ko NU, Amin-Hanjani S, Chou S-Y, Cruz-Flores S, Dangayach NS, Derdeyn CP, et al. 2023 guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023;54(7):e314-e370.
doi pubmed - Thayabaranathan T, Kim J, Cadilhac DA, Thrift AG, Donnan GA, Howard G, Howard VJ, et al. Global stroke statistics 2022. Int J Stroke. 2022;17(9):946-956.
doi pubmed pmc - Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31-S34.
doi pubmed pmc - https://plataformabrasil.saude.gov.br.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604-607.
doi pubmed - R Development Core Team. R Software. R A Lang. 2021. Environ Stat Comput.
- Rodrigues D, Dias CV, Heleno B. Como responder a duvidas clinicas. Rev Port Clinica Geral. 2019;35:155-166.
doi - Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 2016;87(11):1118-1123.
doi pubmed pmc - Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76(5):588-597.
doi pubmed pmc - Von Steinkirch C, Kato MVF, Chyla MM, et al. Avaliacao dos Aneurismas Intracranianos Tratados no Instituto de Neurologia de Curitiba. JBNC - J Bras Neurocir. 2018;28:159-166.
doi - Santos LB dos, Waters C. Perfil dos pacientes submetidos a neurocirurgia para tratamento de aneurismas intracranianos. Arq Medicos dos Hosp e da Fac Ciencias Medicas da St Casa Sao Paulo. 2018;63:1.
doi - Fuentes AM, Stone McGuire L, Amin-Hanjani S. Sex differences in cerebral aneurysms and subarachnoid hemorrhage. Stroke. 2022;53(2):624-633.
doi pubmed - Xia C, Hoffman H, Anikpezie N, Philip K, Wee C, Choudhry R, Albright KC, et al. Trends in the incidence of spontaneous subarachnoid hemorrhages in the United States, 2007-2017. Neurology. 2023;100(2):e123-e132.
doi pubmed pmc - Galvao J, Lima DD de, Haas LJ. Prevalencia de aneurismas cerebrais incidentais entre homens e mulheres. Saude e Pesqui. 2020;13:309-316.
doi - Fiorio CE, Cesar CLG, Alves MCGP, Goldbaum M. Prevalencia de hipertensao arterial em adultos no municipio de Sao Paulo e fatores associados. Rev Bras Epidemiol. 2020;23:E200052.
doi - O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761-775.
doi pubmed - Bonilha L, Marques EL, Carelli EF, Fernandes YB, Cardoso AC, Maldaum MV, Borges G. Risk factors and outcome in 100 patients with aneurysmal subarachnoid hemorrhage. Arq Neuropsiquiatr. 2001;59(3-B):676-680.
doi pubmed - G. B. D. Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795-820.
doi pubmed pmc - Neifert SN, Chapman EK, Martini ML, Shuman WH, Schupper AJ, Oermann EK, Mocco J, et al. Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res. 2021;12(3):428-446.
doi pubmed - Coutinho ESF, Cunha GM da. Conceitos basicos de epidemiologia e estatistica para a leitura de ensaios clinicos controlados. Rev Bras Psiquiatr. 2005;27:146-151.
doi - Ohman J, Heiskanen O. Timing of operation for ruptured supratentorial aneurysms: a prospective randomized study. J Neurosurg. 1989;70(1):55-60.
doi pubmed - de Gans K, Nieuwkamp DJ, Rinkel GJ, Algra A. Timing of aneurysm surgery in subarachnoid hemorrhage: a systematic review of the literature. Neurosurgery. 2002;50(2):336-340; discussion 340-332.
doi pubmed - Oudshoorn SC, Rinkel GJ, Molyneux AJ, Kerr RS, Dorhout Mees SM, Backes D, Algra A, et al. Aneurysm treatment <24 versus 24-72 h after subarachnoid hemorrhage. Neurocrit Care. 2014;21(1):4-13.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
