| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Case Report
Volume 2, Number 2, April 2012, pages 54-58
Blindness or Agnosia: Review of Posterior Cortical Atrophy and a Difficult Case
Bo Biering-Sorensena, c, Moa Gustafssona, Jesper Gyllenborga, Ian Lawb, Peter Hogha
aDepartment of Neurology, Roskilde University Hospital, Denmark
bDepartment of Clinical Physiology, Nuclear Medicine and PET, Copenhagen University Hospital Rigshospitalet, Denmark
cCorresponding author: Bo Biering-Sorensen, Department of Neurology, Roskilde, Copenhagen University Hospital, Kogevej 7-13, DK-4000, Roskilde, Denmark
Manuscript accepted for publication April 6, 2012
Short title: Blindness or Agnosia
doi: https://doi.org/10.4021/jnr91w
| Abstract | ▴Top |
The Objective was to briefly review the rare condition Posterior Cortical Atrophy (PCA) and demonstrate a difficult case suffering from both visual disturbances due to glaucoma and visual agnosia due to PCA. The short review was made by Pub Med search including “Posterior Cortical Atrophy” and the reference lists from the obtained articles were scrutinized for relevant literature not retrieved from PubMed. PCA is a slowly progressing neurodegenerative disorder that is very disabling due to disturbances of higher visual functions, although memory might be relatively preserved until late in the course. Some previous authors have underscored, that to diagnose a patient with PCA one must rule out that the patient is suffering from an eye disease. We demonstrate a difficult case suffering from both glaucoma and PCA. The diagnostic characteristics for PCA are described, and neuroimaging including positron emission tomography (PET) with the amyloid binding ligand (11C) Pittsburgh compound B (PiB), and results from treatment of PCA are presented. Caution should be advised when patients with impaired visual acuity develop signs of cognitive dysfunction, and a full neuropsychological examination should be performed whenever there is doubt of the significance of visual impairment and impaired visuospatial functions.
Keywords: Posterior cortical atrophy; Visual agnosia; Glaukoma; Cholinesterase inhibitor; PiB-PET
| Introduction | ▴Top |
Posterior cortical atrophy (PCA) was first described by Benson in 1988 [1]. It is a rare, slowly progressing neurodegenerative disorder in which primarily the occipito-parietal-temporal junction is affected. The disease is initially dominated by disturbances in higher visual functions including object agnosia, prosopagnosia, alexia, agraphia, environmental disorientation and some aspects of Balint’s or Gerstmann’s syndrome. Patients often demonstate constructional, dressing and ideomotor apraxia, left hemineglect and homonym lateral hemianopsia (more often right than left). Language, memory, insight, and judgement remain relatively preserved until late in the course [1, 2].
Due to its characteristic neuropsychological deficits and its typical features in structural and functional imaging, PCA is possibly to be regarded as a distinct clinical syndrome with its own diagnostic criteria and specific therapeutic interventions [3, 4], although it may be argued that PCA could simply be an unusual presentation of Alzheimers Disease (AD), often with young onset.
The exact prevalence and incidence of PCA is unknown. The mean age of symptom onset in PCA patients is around 60 years [5]. The course of the illness is typically from 8 - 12 years from the onset of symptoms to death [2].
A characteristic neuroradiological (MRI) finding is atrophy of occipital, parietal and posterior temporal lobes. Compared to AD, PCA shows greater loss to the primary visual cortex, visual association cortices and right parietal lobes, but less severe loss of the left medial temporal lobe. While atrophy in typical AD seems to be left hemisphere predominant, atrophy in PCA is primarily seen in the right hemisphere [4]. On functional imaging (SPECT, PET) deficits of perfusion and metabolism in both lateral and medial parietal associative cortex are demonstrated, with variable involvement of the adjacent temporal and occipital associative cortex. Some studies have shown involvement of the frontal lobes, possibly related to deafferenting of areas related to the control of eye movements [6]. Compared with typical AD, PCA presents selective hypometabolism and hypoperfusion in the occipito-parietal region, the right hemisphere is generally much worse affected than the left [6, 7]. Diffusion tensor imaging (DTI) and the association with neuropsychological variables suggest that PCA starts as a distinct clinical syndrome but subsequently turns into a final pathway shared with AD [3].
Looking at Apolipoprotein E (ApoE) genotypes, tau haplotypes, and neuropathologic findings, neither ApoE epsilon4 nor tau haplotype frequencies are different from typical AD. When autopsied, most PCA patients have AD pathology, but compared to typical AD, the neuritic plaques and neurofibrillary tangle densities are significantly higher in Brodmann areas 17 and 18 and significantly lower in the hippocampus, temporal and prefrontal cortex [5]. Because of these pathological findings PCA has been regarded as a focal form of AD [8].
| Case Report | ▴Top |
This 67-year-old, right-handed woman, previously a canteen assistant, started aged 64 years with complaints of insidious onset of difficulties in orientation. She was referred to an ophthalmologist who found high intraocular pressure and loss of peripheral vision and diagnosed her with bilateral glaucoma (Fig. 1). The patient was treated for glaucoma and during the following two years a concentric narrowing of peripheral vision remained steady, but the spatial orientation ability gradually worsened. An MRI showed central atrophy mostly on the right side (Fig. 2A). She was referred to a neurologist in 2009, due to progressive difficulties in reading, writing and remembering numbers. She could not find her kitchen equipment but was able to put them back in place when it was handed to her. She was not able to operate a washing machine, oven or a telephone. She did not leave the apartment, without an accompanying person, because of difficulties in finding her way even in familiar surroundings.
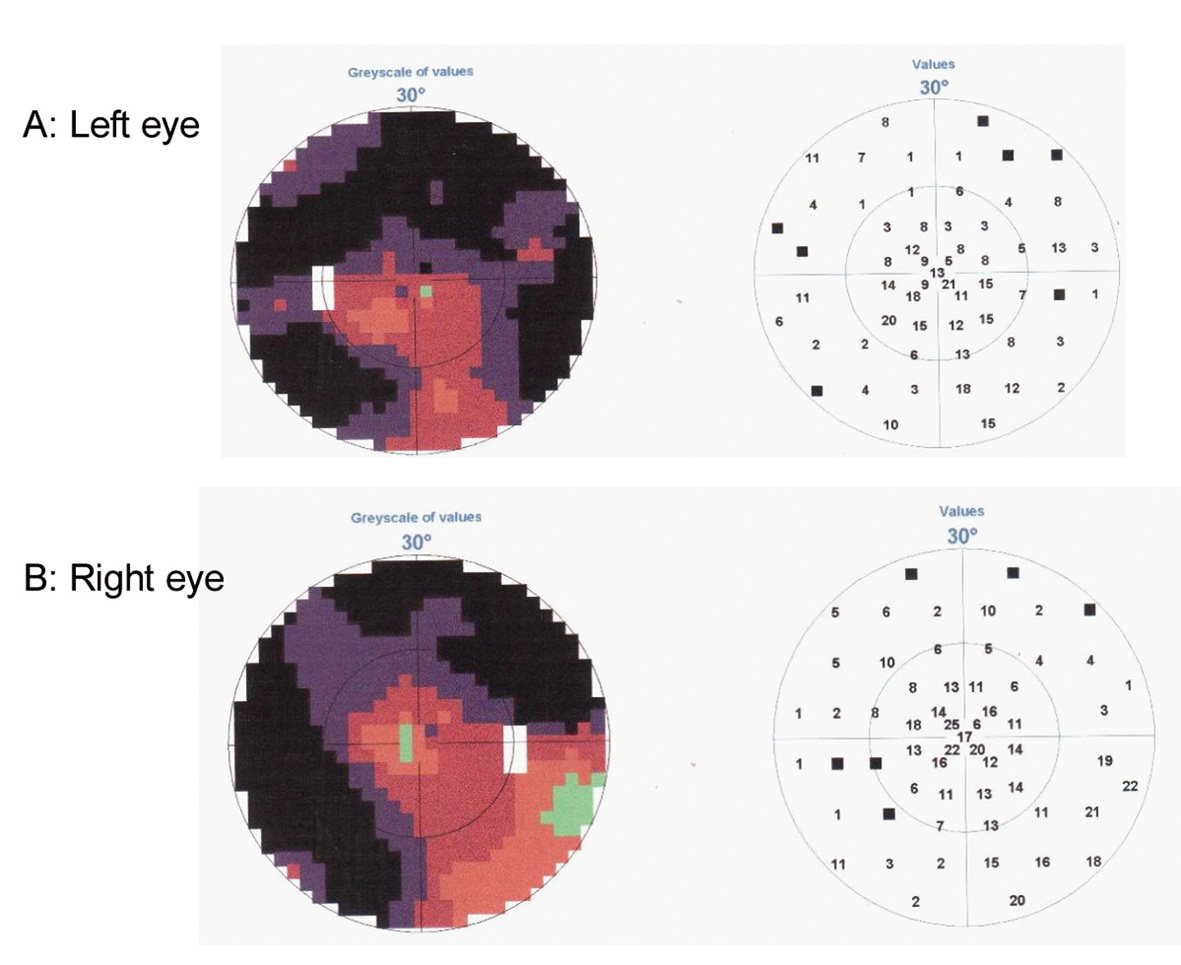 Click for large image | Figure 1. Perimetry test demonstrating loss of peripheral vision on Left eye (A) and Right eye (B). |
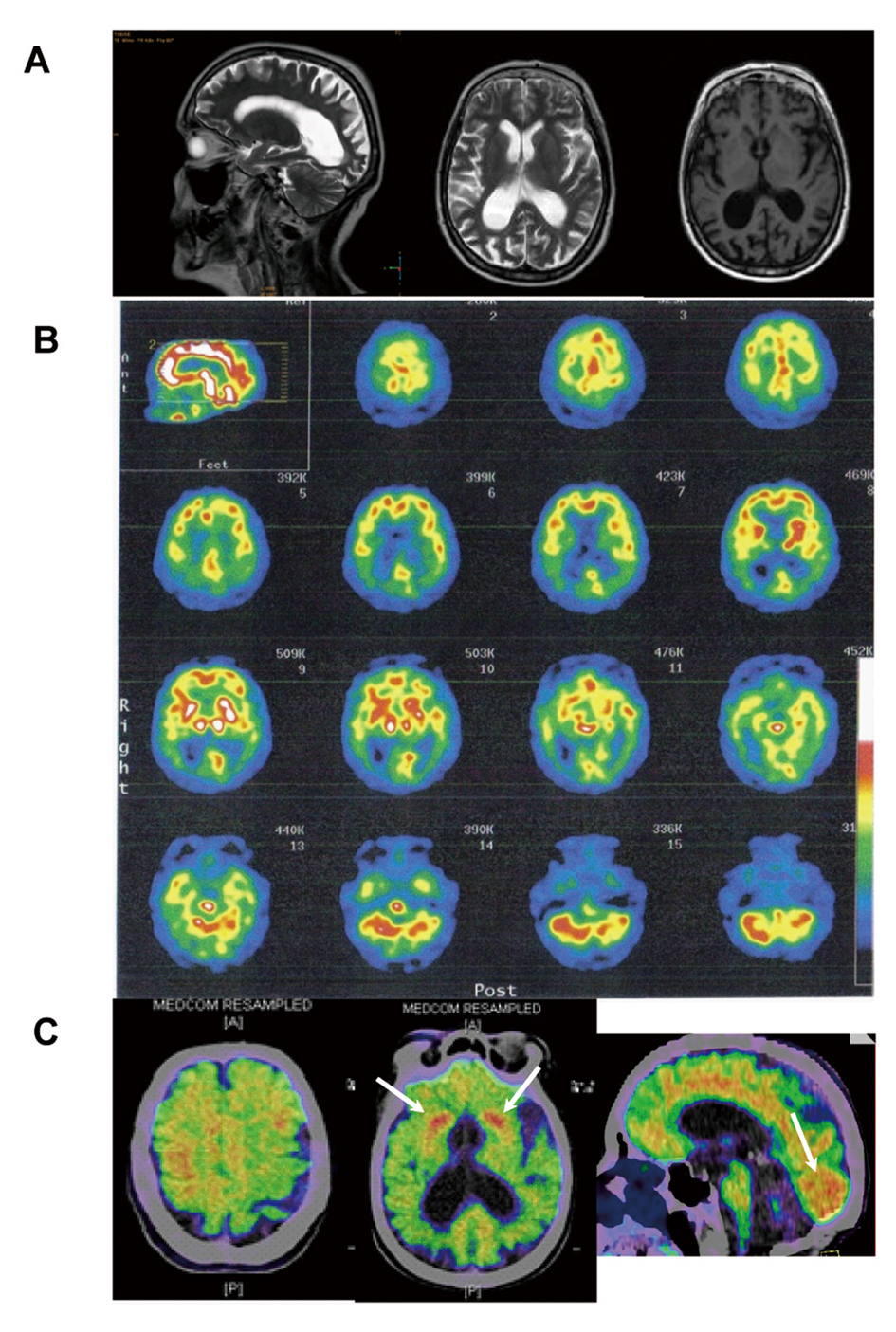 Click for large image | Figure 2. T2- (sagittal and horizontal) and T1-weighted (horizontal) MRI images demonstrating marked posterior cortical atrophy (A), 99mTc-HMPAO SPECT showing prominent parieto-occipital reduction of rCBF (B) and (C-11)PiB-PET (Pittsburgh Compound B) illustrating marked deposition of amyloid in grey matter, particularly pronounced in occipital lobes and in striatum bilaterally (C, arrows). |
There was no family history of dementia and her medical history was unremarkable except for venous insufficiency of the lower extremities and glaucoma. General neurological examination suggested significant cognitive dysfunction and signs of visual agnosia. The Mini-mental state examination (MMSE) score was 17/30 and the Addenbrooke’s Cognitive Examination score was 54/100. The Geriatric Depression Scale score was not indicating depression. Neuropsychometry revealed severe visual object agnosia. She could not recognize the eraser or a teaspoon when she looked at them, but could identify them immediately as soon as she took them in her hand. She had right-left disorientation and signs of simultane agnosia, which was reflected in her difficulty in perceiving more than one part of visual information at a time. She demonstrated prosopagnosia, with inability to recognize familiar faces. After consultation she did not recognize her own husband in the waiting room, although he was standing right in front of her. She had apraxia, i.e. she could not demonstrate the correct usage of a comb or a toothbrush as well as agraphia, alexia and acalculia. Compared to these severe findings, memory was relatively preserved, especially with reference to the massive and dominant impairment of episodic memory which is generally found in AD. Simultaneously, the patient had massive problems with her learning ability.
The patient was referred to a SPECT ((99mTc)-HMPAO) of the brain, which demonstrated severe bilateral temporal, parietal and occipital hypoperfusion (Fig. 2B). The CSF analysis showed one AD biomarker out of three (reduced amyloid beta 42). Other laboratory findings were normal.
Previously casuistically reported effect of cholinesterase inhibitor drugs on visuoperceptual and visuospatiale functions in PCA lead to treatment with the cholinesterase inhibitor Exelon (patch at 4.5 mg/day), dose was increased to 9.5 mg/day after three weeks.
On clinical follow-up six months after initiation of treatment, activities of daily living and MMSE score were stable.
Nine months after the treatment initiation the activities of daily living deteriorated. She had progressing dressing apraxia and neuropsychometry showed clear deterioration of the visuoperceptual and visuospatial functions in all tested parameters except for the previously signs of simultanagnosia and right-left disorientation. Learning capacity was unchanged (possibly slightly better) and the patient had unaltered reduced span for numbers and sentences (Table 1).
 Click to view | Table 1. Neuropsychometry |
A (11C)PiB-PET-PET scan, made 10 months after initiation of treatment, showed increased cortical and subcortical grey matter amyloid deposits, that were unusually pronounced bilaterally in the occipital lobes and in the striatum (Fig. 2C).
| Discussion | ▴Top |
The patient fulfils the clinical description and neuroimaging characteristics of PCA. Although already severely affected by the condition at the time of referral, she kept on attributing her symptoms to her vision difficulties due to glaucoma. In this case it was evidently crucial to recognise the symptoms of visual agnosia.
Only very few studies have casuistically examined the response to cholinesterase inhibitors in PCA, anticipated by the neuropathological similarities between AD and PCA. A case report by Kim E. 2005 [9], showed that cholinesterase inhibitor administration led to amelioration of symptoms, with moderate improvements in visuoconstructive function, simultanagnosia and attention six months after treatment initiation, activities of daily living were moderately improved.
In our case a clear deterioration of the visuoperceptual and visuospatial functions was documented, while memory was unchanged 9 months after initiation of Exelon treatment.
Only few previous studies on (11C)PiB-PET in PCA patients are published. Two recent studies reported accumulation of on (11C)PiB especially in the posterior areas and one with more intense uptake on the right side [8, 10]. However another resent study suggests that in mild to moderate disease stage there is no difference in the distribution of fibrillar Aβ pathology between PCA and AD [7]. Our patient showed unusually pronounced amyloid binding bilaterally in occipital gray matter areas and in the striatum. Strong retention of (C-11)PiB in the striatum has previously been shown in patients with early-onset Alzheimer's disease as a result of the M139V presenilin-1 mutation [11]. Amyloid binding in the occipital lobes is usually low to moderate. However, strong retention in the occipital lobes has been associated with cerebral amyloid angiopathy [12].
In conclusion, caution should be advised when patients with impaired visual acuity develop signs of cognitive dysfunction, and a full neuropsychological examination should be performed whenever there is doubt of the significance of visual impairment and impaired visuospatial functions.
Acknowledgments
We thank the patient and her family for their cooperation.
Conflict of Interest and Sources of Funding
Dr. Biering-Sørensen, Reports no disclosures. Moa Gustafsson, Reports no disclosures. Dr. Gyllenborg, Reports no disclosures. Dr. Hogh is primary investigator on clinical trials on donepezil slow release (EISAI) and H6L-MC-LFAN (Eli Lilly) as well as GE-067005 (GE Healthcare). He has been active as chairman, meeting coordinator and invited speaker at conferences sponsored by Eli Lilly, Pfizer, Novartis and Lundbeck (single honorary). Dr. Law, Reports no disclosures.
| References | ▴Top |
- Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45(7):789-793.
pubmed doi - Croisile B. Benson’s syndrome or Posterior Cortical Atrophy. Orphanet Encyclopedia.September 2004:1-4.
- Duning T, Warnecke T, Mohammadi S, Lohmann H, Schiffbauer H, Kugel H, Knecht S, et al. Pattern and progression of white-matter changes in a case of posterior cortical atrophy using diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2009;80(4):432-436.
pubmed doi - Whitwell JL, Jack CR, Jr., Kantarci K, Weigand SD, Boeve BF, Knopman DS, Drubach DA, et al. Imaging correlates of posterior cortical atrophy. Neurobiol Aging. 2007;28(7):1051-1061.
pubmed doi - Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168-1174.
pubmed - Nestor PJ, Caine D, Fryer TD, Clarke J, Hodges JR. The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer's disease) with FDG-PET. J Neurol Neurosurg Psychiatry. 2003;74(11):1521-1529.
pubmed doi - Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O'Neil JP, Janabi M, Yen IV, et al. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76(21):1789-1796.
pubmed doi - Formaglio M, Costes N, Seguin J, Tholance Y, Le Bars D, Roullet-Solignac I, Mercier B, et al. In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings. J Neurol. 2011;258(10):1841-1851.
pubmed doi - Kim E, Lee Y, Lee J, Han SH. A case with cholinesterase inhibitor responsive asymmetric posterior cortical atrophy. Clin Neurol Neurosurg. 2005;108(1):97-101.
pubmed doi - Kambe T, Motoi Y, Ishii K, Hattori N. Posterior cortical atrophy with [11C] Pittsburgh compound B accumulation in the primary visual cortex. J Neurol. 2010;257(3):469-471.
pubmed doi - Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, Bi W, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27(23):6174-6184.
pubmed doi - Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62(3):229-234.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
