| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 7, Number 3, June 2017, pages 39-45
Transcranial Light Alters Melanopsin and Monoamine Production in Mouse (Mus musculus) Brain
Antti Flyktmana, c, d, Toni Jernforsa, Satu Manttarib, Juuso Nissilaa, c, Markku Timonenc, Seppo Saarelaa
aDepartment of Ecology and Genetics, University of Oulu, PO Box 3000, FIN-90014, Oulu, Finland
bFinnish Institute of Occupational Health, Aapistie 1, FIN-90220 Oulu, Finland
cCenter for Life Course Health Research, University of Oulu, PO Box 5000, FIN-90014, Oulu, Finland
dCorresponding Author: Antti Flyktman, Department of Ecology and Genetics, University of Oulu, PO Box 3000, FIN-90014, Oulu, Finland
Manuscript accepted for publication April 10, 2017
Short title: Transcranial Light Alters Circadian Molecules
doi: https://doi.org/10.14740/jnr427w
| Abstract | ▴Top |
Background: The mammalian circadian system sets a rhythm for the appropriate occurrence of physiological and behavioral phenomena during a 24-h period. Since the duration of the circadian system is usually less or more than 24 h, it must be entrained regularly and light is the governing stimulus of the rhythm. The target for light stimulus is the master circadian clock, which is located in the suprachiasmatic nucleus in the hypothalamus. One of the key molecules transmitting light information and entraining the clock is melanopsin (OPN4), a G protein-coupled molecule that is found most abundantly in the retina and brain. Although light stimulus is usually mediated through the eyes, light has an ability to penetrate the skull. Here, we present the effect of transcranial light illumination on OPN4 and serotonin expression in the mouse brain.
Methods: Male mice were randomly assigned to a control group, morning-light group and evening-light group, and animals were illuminated transcranially five times a week for 8 min for a total of 4 weeks. The concentrations of OPN4 and monoamines were analyzed with Western blot and high-performance liquid chromatography (HPLC) techniques, respectively.
Results: Our results show that transcranial light illumination increases the amount of OPN4 in the hypothalamus and cerebellum. Additionally, the production of serotonin in the cortex was shown to decrease in the morning-light group.
Conclusions: With this study, we provide novel information on the effects of light administration through the skull on transmitters regulating circadian rhythmicity by showing that transcranial light affects molecules involved in circadian rhythmicity.
Keywords: OPN4; Light through skull; Circadian rhythm; Hypothalamus; Cerebellum; Serotonin
| Introduction | ▴Top |
Light is the main signal that entrains the mammalian circadian system. This system is evolved to a set rhythm, in which physiological and behavioral events occur during a 24-h period. Since the circadian clock is a free-running system, and produces a rhythm even when environmental cues are absent [1], it is not exactly 24 h, and thus must be entrained regularly to ensure accordingly timed physiological phenomena [2]. If the light stimulus is diminished or absent, the circadian clock is disturbed and several psychoneurological disorders, such as seasonal affective disorder (SAD) in humans, may occur [3]. Furthermore, mice have also been shown to have SAD-like behavior, when light conditions are irregular [4].
The mammalian master circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Its main purpose is to generate and synchronize the circadian rhythms of peripheral tissues. According to a generally accepted assumption, the only route by which light signals reach the hypothalamus is through the eyes. This hypothesis suggests that intrinsically photosensitive retinal ganglion cells (ipRGCs) gather light information in the retina and project it to the SCN through the retinohypothalamic tract (RHT) [2, 5, 6]. One of the key molecules for retinal phototransduction is melanopsin (OPN4) [6-9], although it is not crucial for these events since multiple types of photoreceptors are capable of transmitting light stimuli [7].
Phototransduction is not necessarily restricted to the RHT. It has been shown that mammalian brain consists of photosensitive opsin proteins also outside the RHT [10-13], and that light penetrates the mammalian skull [14-17]. It has been also proved that light is capable of phosphorylating OPN4, which inhibits the G protein-coupled activation [18], although only some OPN4 gene variants have been shown to affect the responses to light stimulus [19]. Notwithstanding general criticism, we have recently shown that it is possible to stimulate brain opsins by illuminating the brain via ear canals [20].
OPN4 is a part of a large and old family of heptahelical light-sensitive G protein-coupled transmembrane receptors, which consist of a protein constituent and a chromophore. Opsins are expressed mostly in the brain and in the retina of mammals [21, 22]. The opsin-mediated phototransduction mechanism can be divided into two steps: absorption of light by the retinal part of the opsin protein and the subsequent light-initiated photoisomerization from 11-cis to all-trans conformation [23]. This conformational change allows activation of the G protein and the phototransduction cascade [2, 24].
OPN4 was found for the first time in the light-sensitive melanophores in frog skin as well as in the brain SCN and the eye [25]. Furthermore, OPN4 has been found in all animal groups, including mammals, and it functions as a photoisomerase [26, 27]. The functions of OPN4 are very different from the other photosensory opsins [24], since it is also required for non-visual photic responses [28] in the eye. In mammals, OPN4 is the most abundant photopigment of ipRGCs and it is a key molecule in mediating the effects of light on the brain via RHT [29]. OPN4 is an important factor for maintaining circadian rhythms also, although not crucial for entrainment in all mammalian species [7, 30, 31].
Besides opsins, monoamines are also shown to affect circadian rhythmicity. Many external factors influence the monoamine concentration in the hypothalamus, including season, the time of the day, and the intensity of light [32, 33]. Apart from the brain, light also changes the monoamine concentration in the peripheral tissues. The extraneuronal monoamine transporter has been known to be an important factor in a signal transmission chain as an agent transferring monoamine-based information to peripheral tissues [34].
As mentioned above, if the photoperiod of an animal is shortened, certain physiological conditions may occur. Besides the mood alterations, SAD has been shown to increase body weight due to increased carbohydrate foraging and decreased physical activity [35, 36]. Bright light treatment is used as a therapy method for helping SAD symptoms. It decreases body weight, especially due to reduction of body fat, in obese humans regardless of the seasonality trait [37]. Furthermore, it has been shown that rhythm-related gene loci affect long-term changes in energy expenditure [38], but the direct effects of transcranial light on the changes energy expenditure have not yet been studied.
As shown, OPN4 is expressed in the vertebrate brain [25, 27, 39], and opsins can be stimulated transcranially [20]. Additionally, monoamines play a role in light signaling [40], and also e.g. in SAD symptoms [41]. In order to determine whether transcranial light has direct effects on OPN4 and monoamine expression in the brain at different times of the day, we illuminated mice transcranially either in the morning or in the evening. With the results of this study, we are able to show that light through the skull plays a role in activating molecules that are associated with circadian rhythmicity.
| Materials and Methods | ▴Top |
Animals
Thirty (30) adult C3H/HeNHsd rd/rd male mice (Mus musculus, Harlan Laboratories, Venray, The Netherlands) were used in this study, which was approved by the Finnish National Committee for animal experimentation (license number ESAVI/567/04.10.03/2012). The animals were 8 - 10 weeks old at the beginning of the study. The mice used in the study were visually blind. Their rods had degenerated 5 weeks postnatal and cones secondarily, which leads to adult blindness [42], but the ganglion cell layer is functional.
Experimental procedure
All the mice were kept in a 12:12 LD rhythm. The light period of the mice started at 7:00 and finished at 19:00. Mice were randomly assigned to three different groups: control (CONT), morning-light group (ML) and evening-light group (EL). The animals were weighed three times a week. Transcranial light was given via ear canals for 4 weeks, five times a week. Light treatment was given under anesthesia (isofluran, anesthesia 3%, maintenance 1.5%) for 8 min per mouse. The ML group was exposed to light just after the beginning of the light period (treatment time 7:30 - 9:30) simulating the time when mice are typically inactive, and the EL group was exposed just after the beginning of the dark period (treatment time 19:00 - 21:00), simulating the time when mice are typically active [43]. The intensity of the light in the headphones of the light source (Valkee NPT 1000, Valkee Inc., Oulu, Finland) was 2.00 × 1015 photons/cm2/s. We have presented the spectrum of the headphones in our previous article with an intensity peak at approximately 450 nm [20]. There is no significant heat effect caused by the device, since only a non-measurable small fraction of the mechanical heat produced by the light source (totally less than 14.7 mW) is conducted into the ear canal. The CONT group was anesthetized and headphones were inserted, but no light was given. After 4 weeks of light treatment, mice were killed by cervical dislocation, the length and body mass of the animals were measured and samples of cortex, hypothalamus, cerebellum, liver, retina and plasma were collected between 8:00 and 14:00 and stored at -80 °C. Body mass indexes (BMI) were calculated using the general BMI formula: BMI = mass/length2. Masses were described as kilograms and length as meters.
SDS-PAGE and Western blotting
Total protein concentrations of the brain tissue samples were defined according to Bradford [44]. Homogenization buffer (62.5 mM Tris-HCl, pH 6.8), containing leupeptin (1 µg/mL), pepstatin A (1 µg/mL) and PMSF (1 mM) was added to the samples and homogenized (Qiagen TissueLyser, Retsch, Haan, Germany).
Samples with equal amounts of protein (12.06 μg/lane) were loaded in separating gel (4-12%, AmershamTM ECLTM Gel, GE Healthcare Bio-Sciences, Uppsala, Sweden) and electrophoretically separated at 160 V for 60 min. The separated proteins were transferred to a nitrocellulose membrane (0.45 μm, Bio-Rad Laboratories, USA) according to the method of Towbin et al [45].
After electroblotting, the membranes were blocked with 5% non-fat milk powder in TBS for 1 h at room temperature. After washing (3 × 5 min) in TBST (TBS + 0.05% Tween-20), the membranes were incubated in primary antibody for melanopsin (molecular weight 53 kDa, anti-melanopsin antibody, ab65641, Abcam, Cambridge, UK) for 2 h at room temperature. Melanopsin antibody was diluted in TBST (1:500). Then the membranes were washed (3 × 5 min) and incubated with a secondary antibody (1:3,000, Bio-Rad Goat Anti-Rabbit IgG (H+L)-AP-Conjugate, Bio-Rad Laboratories, Hercules, USA) in TBST for 2 h at room temperature. Antibody detection was performed with bromo-4-chloro-3-indolyl phosphate mono-(-toluidinium) salt/ nitro blue tetrazolium (BCIP/NBT) substrate. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, molecular weight 37 kDa) was used as a loading control (GAPDH (14C10) Rabbit mAb, Cell Signaling Technology, Danvers, USA). To measure gel loading, membranes were stripped using stripping buffer (1.5 g glycine, 0.1 g SDS, 1 mL Tween-20 diluted in 100 mL ultrapure water; pH 2.2). Membranes were incubated 10 min with stripping buffer, followed by 2 × 10 min wash with PBS and 2 × 5 min wash with TBST. After washing, the stripped membranes were blotted as before. GAPDH was diluted in TBST-antibody solution (1:1,000) and incubated for 2 h with the primary antibody. Immunoreactive band intensities were analyzed using the VersaDoc imaging system (Bio-RadUSA). Liver was used as a negative control.
Monoamine concentration analysis by high-performance liquid chromatography (HPLC)
Monoamine concentrations were determined by HPLC in plasma and adrenal gland samples, as described by Nieminen et al [46]. The 5-HT samples were analyzed using the same technique. Briefly, brain samples were deproteinized before injection into the HPLC autosampler. The running buffer contained 50 mM NaH2PO4, MetOH and aluminum chlorohydrate in 77:15:8 ratios, respectively (pH 3.22). The running buffer was filtered and 200 mg Na-dodecylsulphate and 77.4 mg Na-EDTA were added to the buffer. The total concentration of base solution was 5 µg/mL in 0.1 M HClO4.
Statistical analysis
In this study, all values are expressed as mean ± standard error (SE). For the amounts of OPN4, monoamines and melatonin, one-way ANOVA was used for comparisons between groups. In case of significant changes, pairwise comparisons between both the test group and CONT group were made using the Tukey posterior test. The length, weight and BMI comparisons between groups were also made with one-way ANOVA and Tukey posterior test. Weight changes within groups were compared using a paired t-test.
| Results | ▴Top |
Melanopsin protein abundance in the mouse hypothalamus and cerebellum
Western blot was used to analyze the abundance of OPN4 in mouse hypothalamus and cerebellum. The used antibody detected a single band of approximately 53 kDa, similar to the predicted protein size of OPN4. In hypothalamus samples, significant differences were observed between control and experimental groups in the OPN4 content. Compared to the CONT group, the amount of OPN4 was 1.72-fold higher in the ML group (P < 0.001), and 1.54-fold higher in the EL group (P < 0.01) (Fig. 1a). The amount of OPN4 in the cerebellum was 1.21-fold higher in the EL group (P < 0.01), but no differences were seen in the ML group compared to controls. Additionally, compared to the ML group, the amount of OPN4 was 1.55-fold higher in the EL group (P < 0.001) (Fig. 1b). A Western blot showing the OPN4 expression in different groups and tissues is represented in Figure 1c.
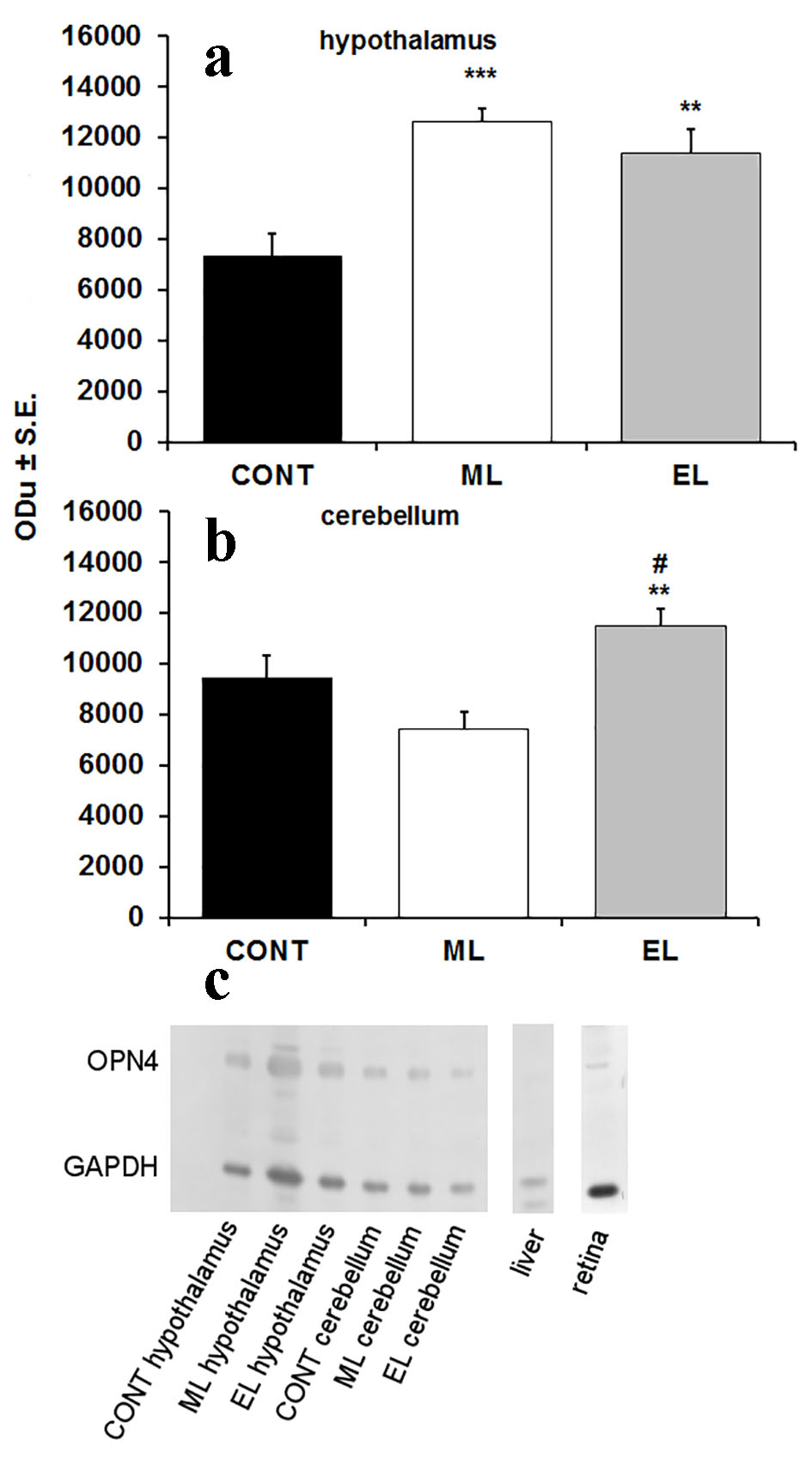 Click for large image | Figure 1. Expression of OPN4 in hypothalamus and cerebellum of mouse brain. (a) A bar graph summarizing results from Western blot analysis showing mean optical density (ODu) of OPN4 in relation to GAPDH in hypothalamus (N = 10 samples per group). **P < 0.01, ***P < 0.001; error bars indicate SE. (b) A bar graph summarizing results from Western blot analysis showing mean optical density (ODu) of OPN4 in relation to GAPDH in cerebellum (N = 10 samples per group). **P < 0.01; error bars indicate SE. # marks the significance compared to ML group, ##P < 0.001. (c) A representative Western blot membrane showing expression of OPN4 with a molecular weight of 53 kDa. GAPDH was used as a loading control with a molecular weight of 37 kDa. CONT: control group; ML: morning-light group; EL: evening-light group. |
Monoamines in the mouse brain
The amounts of adrenaline, noradrenaline, dopamine and serotonin in mouse cortex, hypothalamus and cerebellum were analyzed using the HPLC method. Melatonin concentrations were analyzed from plasma samples. The amount of serotonin in the cortex was 0.66-fold lower (P < 0.01) in ML mice compared to the controls (Fig. 2a), but there were no other differences between the experimental group monoamines and the control group monoamines (Fig. 2b, c). There was no detectable amount of adrenaline in the brain samples. There was no measurable amount of melatonin in any groups. No changes were seen in BMIs between groups at the end of the study.
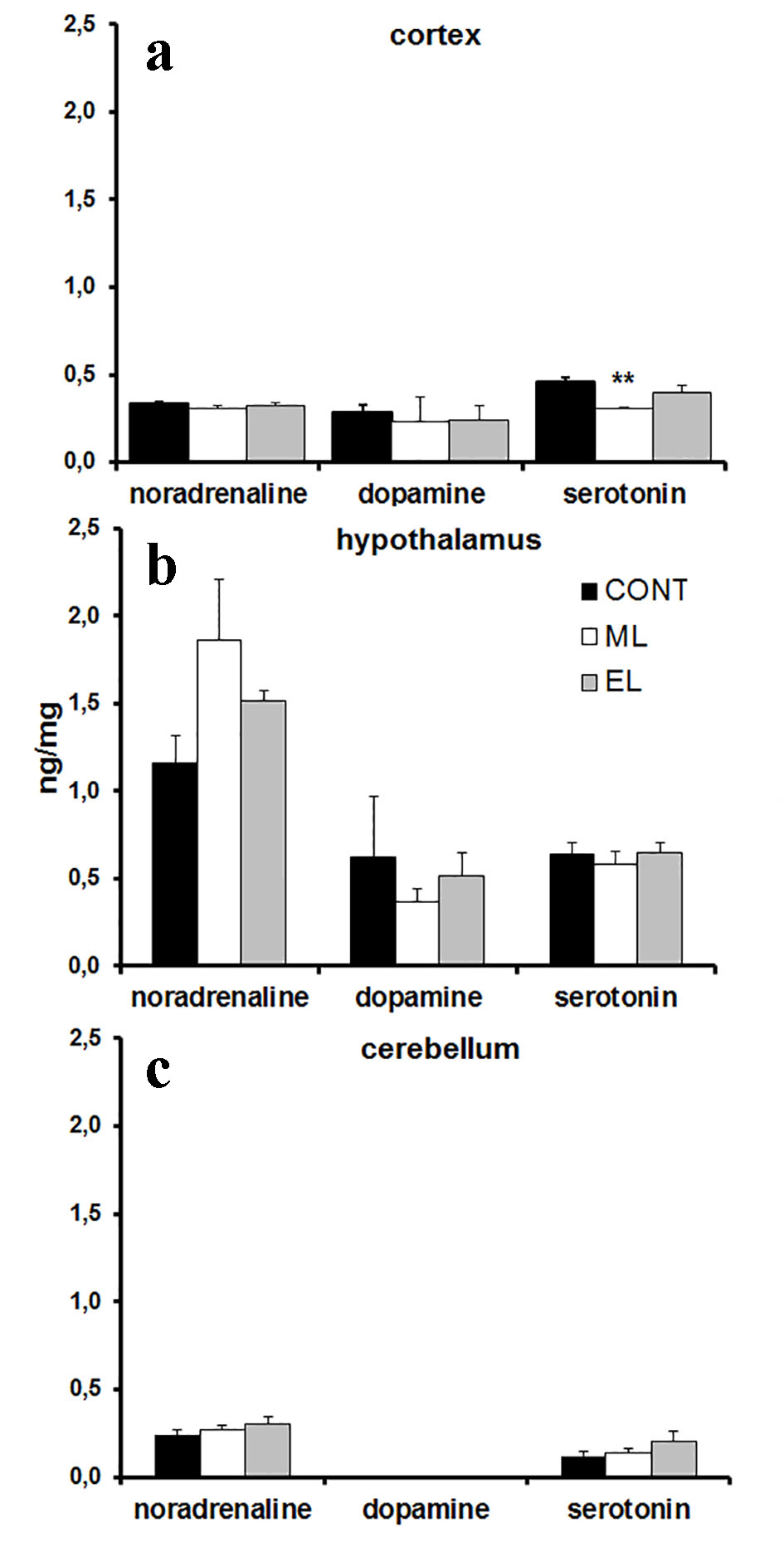 Click for large image | Figure 2. Monoamine concentrations in mouse cortex, hypothalamus and cerebellum. (a) A bar graph summarizing results from HPLC analysis showing mean ng/mg in cortex. N = 10 samples per group. **P < 0.01. (b) A bar graph summarizing results from HPLC analysis showing mean ng/mg in hypothalamus. N = 8 - 10 samples per group. (c) A bar graph summarizing results from HPLC analysis showing mean ng/mg in cerebellum. N = 8 - 10 samples per group. CONT: control group; ML: morning-light group; EL: evening-light group. Error bars indicate SE. |
| Discussion | ▴Top |
In this study, we describe the direct effects of transcranial light on OPN4 and monoamine expression in the mammalian brain. We were also able to show the connection between transcranial light and circadian rhythmicity, since the results of the expression level of OPN4 in the cerebellum differed between the test groups. There were significant differences both in OPN4 and 5-HT expression levels related to the time when the light was administered. This study showed that the amount of OPN4 increased significantly in transcranially illuminated mice when compared to controls. Furthermore, the 5-HT production in the cortex decreased when mice were transcranially illuminated in the morning.
Based on the findings of this study, light-activated molecules in the brain can be stimulated with transcranial light. In the hypothalamus, the amount of OPN4 in transcranially illuminated mice was higher than in the controls, and the amount of OPN4 was higher regardless of the duration of illumination. The commonly acknowledged and best-known route for light-mediated effects is through the eye, where OPN4-containing ipRGCs gather light information and deliver it to the SCN via RHT [6, 47, 48]. Besides the retina, OPN4 is also found in the vertebrate brain [25, 39] along with other opsins [10, 12, 13, 39]. Additionally, OPN4 is considered to be an important molecule in adjusting the master clock and entraining the circadian rhythm [7-9, 28], and light has been shown to act as a direct modulator for OPN4 actions [30]. Therefore, it is reasonable to hypothesize that transcranial light entrains the circadian system by enhancing OPN4 production in the hypothalamus. Since OPN4 is known to have an important role in light transmission through the eyes, and on the other hand, OPN4 is shown to be expressed in the hypothalamus [27], it may be capable of activating circadian signaling pathways directly by transcranially penetrated light, although we acknowledge that there might be some light transmitted through the transcranially illuminated ocular photoreceptors [49, 50]. Even though the absorption maximum of our light source (450 nm) [20] was not optimal for the OPN4 expression maximum (476 - 484 nm) [51, 52], we were able to obtain significant results in this study.
The time of the illumination had an effect on the amount of OPN4 in the cerebellum also. We found that the expression of OPN4 was significantly higher in the EL group, but no differences were found in the ML group compared to controls. Moreover, the amount of OPN4 was significantly higher in the EL group when compared to the ML group. OPN4 has been found in the vertebrate cerebellum [27, 39]. Whether light has a direct effect on its expression in the cerebellum has not yet been clarified. It has also been shown that the cerebellum is involved in maintaining the circadian rhythm in rodents through clock gene regulation [53, 54], and OPN4 resets the circadian clock through the activation of clock gene Per1 [55]. Therefore, we must consider the possibility that OPN4 may affect the circadian rhythm through clock gene activation in the cerebellum. The amount of OPN4 mRNA in the brain is highest in the late subjective night in avian species [39]. Our results suggest a similar occurrence in protein level in mouse cerebellum, as we know that mice are nocturnal animals, and the amount of OPN4 was highest in the EL group. Furthermore, recent research by Kumar et al hypothesizes that myelinated fibers may be capable to transmit light information in the brain [56]. If true, transcranial light may have also direct effect on cerebellum through these fibers.
Transcranial illumination decreased the amount of serotonin in the cortex of ML group mice compared to controls. The function of 5-HT is regulated by monoamine oxidase A (MAO-A), which is an important factor in metabolizing monoamines [57]. In general, if the amount of MAO-A increases, the amount of serotonin decreases [58]. There was a similar pattern in cortical noradrenaline and dopamine in the ML group also, although the changes were not statistically significant. Additionally, the changes in monoamine concentrations may refer to circadian rhythm disturbations [59]. It should be remembered that mice are nocturnal animals and the ML group was illuminated in the beginning of their resting state, which might disturb the circadian rhythm and stress the animals. We have previously published results regarding the effects of transcranial light on monoamine concentrations in plasma and adrenal gland samples [20]. We showed that the levels of dopmanine and noradrenaline increased, and the levels of adrenaline decreased with transcranially illuminated mouse [20]. These results suggest that the experiment created long-term stress effects, which may be caused by the actual experiment or the disturbations in the circadian rhythm. Moreover, the serotonin concentration is shown to peak at the end of the light phase in rodent brain [60-62]. Therefore, we must consider the possibility that the sample collection time was not optimal for serotonin concentration levels in this study.
Owing to our study design, we cannot be sure about the causal relationship between OPN4 and 5-HT concentration. However, based on the results of this study, it is reasonable to hypothesize that the change in cortical 5-HT levels may be linked to the increased OPN4 expression in the brain. OPN4 transfers visual information to the thalamo-cortical pathway, which is involved in many events in the brain [63]. If OPN4 regulates this pathway, it may also affect cortical functions by decreasing 5-HT production in the cortex of ML group mice. However, the level of knowledge regarding the relationship between 5-HT and OPN4 is still low, and the relationship between these two compounds should be further studied. In this connection, it has also to be reminded that another monoamine, i.e., DA has earlier been shown to regulate OPN4 expression in ganglion cells [40]. Also, given that OPN4 gene variants have earlier been shown to be associated in SAD [19], the putative OPN4 mediated treatment effect of transcranial bright light on SAD remains also to be investigated.
In conclusion, this is the first study to show that transcranial light has a significant effect on OPN4 expression in the mouse brain. We were also able to alter the serotonin levels in the cortex with transcranial illumination. This research indicates that molecules influenced by circadian rhythmicity in the brain can be stimulated by light through the skull via ear canals. We only studied the changes in OPN4 expression at the protein level. Therefore the changes at the OPN4 mRNA level would be of interest in future studies, although it is already known that the levels of mRNA and protein have similar pattern in normal lighting conditions in the rodent retina [64]. Additionally, with the used methods, we cannot say if the changes in OPN4 levels are caused by expression level changes inside the cells or by the total amount of OPN4 expressing cells. It would also be important to study closely, whether the changes in OPN4 levels are caused by changes in monoamine levels and vice versa.
Acknowledgments
We acknowledge laboratory technicians Mr Matti Rauman for his contribution of modifying the Valkee headset, and Mrs Marja-Liisa Martimo-Halmetoja and Mrs Minna Orrevetelainen for their contributions in laboratory analyses.
Funding Support
This work was supported by The Northern Ostrobothnia Health District, Valkee Inc., and Alfred Kordelinin foundation (grant number 150182).
Competing Interests
Antti Flyktman has received a small grant from Valkee Inc., Juuso Nissila is a minor shareholder of Valkee Inc. Toni Jernfors, Satu Manttari, Markku Timonen and Seppo Saarela have no competing interests in relation to this study.
| References | ▴Top |
- Toh KL. Basic science review on circadian rhythm biology and circadian sleep disorders. Ann Acad Med Singapore. 2008;37(8):662-668.
pubmed - Bellingham J, Foster RG. Opsins and mammalian photoentrainment. Cell Tissue Res. 2002;309(1):57-71.
doi pubmed - Vandewalle G, Hebert M, Beaulieu C, Richard L, Daneault V, Garon ML, Leblanc J, et al. Abnormal hypothalamic response to light in seasonal affective disorder. Biol Psychiatry. 2011;70(10):954-961.
doi pubmed - LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594-598.
doi pubmed - Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070-1073.
doi pubmed - Bailes HJ, Lucas RJ. Melanopsin and inner retinal photoreception. Cell Mol Life Sci. 2010;67(1):99-111.
doi pubmed - Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298(5601):2211-2213.
doi pubmed - Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307(5709):600-604.
doi pubmed - Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213-2216.
doi pubmed - Blackshaw S, Snyder SH. Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19(10):3681-3690.
pubmed - Wada Y, Okano T, Adachi A, Ebihara S, Fukada Y. Identification of rhodopsin in the pigeon deep brain. FEBS Lett. 1998;424(1-2):53-56.
doi - Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, Ebihara S, et al. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci U S A. 2010;107(34):15264-15268.
doi pubmed - Nissila J, Manttari S, Sarkioja T, Tuominen H, Takala T, Timonen M, Saarela S. Encephalopsin (OPN3) protein abundance in the adult mouse brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198(11):833-839.
doi pubmed - Brunt EEV, Shepherd MD, Wall JR, Ganong WF, Clegg MT. Penetration of light into the brain of mammals. Ann N Y Acad Sci. 1964;117:217-224.
doi - Campbell SS, Murphy PJ, Suhner AG. Extraocular phototransduction and circadian timing systems in vertebrates. Chronobiol Int. 2001;18(2):137-172.
doi pubmed - Persinger MA, Dotta BT, Saroka KS. Bright light transmits through the brain: Measurement of photon emissions and frequency-dependent modulation of spectral electroencephalographic power. World J Neurosci. 2013;3:10-16.
doi - Sun L, Perakyla J, Kovalainen A, Ogawa KH, Karhunen PJ, Hartikainen KM. Human Brain Reacts to Transcranial Extraocular Light. PLoS One. 2016;11(2):e0149525.
doi pubmed - Blasic JR, Jr., Lane Brown R, Robinson PR. Light-dependent phosphorylation of the carboxy tail of mouse melanopsin. Cell Mol Life Sci. 2012;69(9):1551-1562.
doi pubmed - Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114(1-3):279-285.
doi pubmed - Flyktman A, Manttari S, Nissila J, Timonen M, Saarela S. Transcranial light affects plasma monoamine levels and expression of brain encephalopsin in the mouse. J Exp Biol. 2015;218(Pt 10):1521-1526.
doi pubmed - Davies WL, Foster RG, Hankins MW. Focus on molecules: melanopsin. Exp Eye Res. 2012;97(1):161-162.
doi pubmed - Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW, Robinson PR. Shedding new light on opsin evolution. Proc Biol Sci. 2012;279(1726):3-14.
doi pubmed - Terakita A. The opsins. Genome Biol. 2005;6(3):213.
doi pubmed - Bellingham J, Whitmore D, Philp AR, Wells DJ, Foster RG. Zebrafish melanopsin: isolation, tissue localisation and phylogenetic position. Brain Res Mol Brain Res. 2002;107(2):128-136.
doi - Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95(1):340-345.
doi pubmed - Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600-605.
pubmed - Nissila JS, Manttari SK, Sarkioja TT, Tuominen HJ, Takala TE, Kiviniemi VJ, Sormunen RT, et al. The distribution of melanopsin (OPN4) protein in the human brain. Chronobiol Int. 2017;34(1):37-44.
doi pubmed - Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301(5632):525-527.
doi pubmed - Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433(7027):745-749.
doi pubmed - Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, et al. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol. 2009;7(6):e1000125.
doi pubmed - Tsunematsu T, Tanaka KF, Yamanaka A, Koizumi A. Ectopic expression of melanopsin in orexin/hypocretin neurons enables control of wakefulness of mice in vivo by blue light. Neurosci Res. 2013;75(1):23-28.
doi pubmed - Khan IA, Joy KP. Differential effects of photoperiod and temperature on hypothalamic monoaminergic activity in the teleost Channa punctatus (Bloch). Fish Physiol Biochem. 1990;8(4):291-297.
doi pubmed - Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14(6):3635-3642.
pubmed - Haag C, Berkels R, Grundemann D, Lazar A, Taubert D, Schomig E. The localisation of the extraneuronal monoamine transporter (EMT) in rat brain. J Neurochem. 2004;88(2):291-297.
doi pubmed - Rosenthal NE, Genhart M, Jacobsen FM, Skwerer RG, Wehr TA. Disturbances of appetite and weight regulation in seasonal affective disorder. Ann N Y Acad Sci. 1987;499:216-230.
doi pubmed - Winkler D, Pjrek E, Praschak-Rieder N, Willeit M, Pezawas L, Konstantinidis A, Stastny J, et al. Actigraphy in patients with seasonal affective disorder and healthy control subjects treated with light therapy. Biol Psychiatry. 2005;58(4):331-336.
doi pubmed - Danilenko KV, Mustafina SV, Pechenkina EA. Bright light for weight loss: results of a controlled crossover trial. Obes Facts. 2013;6(1):28-38.
doi pubmed - Mirzaei K, Xu M, Qi Q, de Jonge L, Bray GA, Sacks F, Qi L. Variants in glucose- and circadian rhythm-related genes affect the response of energy expenditure to weight-loss diets: the POUNDS LOST Trial. Am J Clin Nutr. 2014;99(2):392-399.
doi pubmed - Bailey MJ, Cassone VM. Melanopsin expression in the chick retina and pineal gland. Brain Res Mol Brain Res. 2005;134(2):345-348.
doi pubmed - Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22(12):3129-3136.
doi pubmed - Neumeister A, Konstantinidis A, Praschak-Rieder N, Willeit M, Hilger E, Stastny J, Kasper S. Monoaminergic function in the pathogenesis of seasonal affective disorder. Int J Neuropsychopharmacol. 2001;4(4):409-420.
doi pubmed - Cavusoglu N, Thierse D, Mohand-Said S, Chalmel F, Poch O, Van-Dorsselaer A, Sahel JA, et al. Differential proteomic analysis of the mouse retina: the induction of crystallin proteins by retinal degeneration in the rd1 mouse. Mol Cell Proteomics. 2003;2(8):494-505.
doi - Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42(4):517-525.
doi - Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254.
doi - Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350-4354.
doi pubmed - Nieminen P, Saarela S, Pyykonen T, Asikainen J, Mononen J, Mustonen AM. Endocrine response to fasting in the overwintering captive raccoon dog (Nyctereutes procyonoides). J Exp Zool A Comp Exp Biol. 2004;301(12):919-929.
doi pubmed - Pickard GE, Sollars PJ. Intrinsically photosensitive retinal ganglion cells. Sci China-Life Sci. 2010;53:58-67.
doi pubmed - Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065-1070.
doi pubmed - Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):502-504.
doi pubmed - Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):505-507.
doi pubmed - Torii M, Kojima D, Okano T, Nakamura A, Terakita A, Shichida Y, Wada A, et al. Two isoforms of chicken melanopsins show blue light sensitivity. FEBS Lett. 2007;581(27):5327-5331.
doi pubmed - Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 2013;280(1759):20122987.
doi pubmed - Rath MF, Rohde K, Moller M. Circadian oscillations of molecular clock components in the cerebellar cortex of the rat. Chronobiol Int. 2012;29(10):1289-1299.
doi pubmed - Rath MF, Rovsing L, Moller M. Circadian oscillators in the mouse brain: molecular clock components in the neocortex and cerebellar cortex. Cell Tissue Res. 2014;357(3):743-755.
doi pubmed - Yamashita T, Ono K, Ohuchi H, Yumoto A, Gotoh H, Tomonari S, Sakai K, et al. Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation. J Biol Chem. 2014;289(7):3991-4000.
doi pubmed - Kumar S, Boone K, Tuszynski J, Barclay P, Simon C. Possible existence of optical communication channels in the brain. Sci Rep. 2016;6:36508.
doi pubmed - Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, et al. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. J Neurosci. 1998;18(17):6914-6927.
pubmed - Holschneider DP, Scremin OU, Huynh L, Chen K, Seif I, Shih JC. Regional cerebral cortical activation in monoamine oxidase A-deficient mice: differential effects of chronic versus acute elevations in serotonin and norepinephrine. Neuroscience. 2000;101(4):869-877.
doi - Iritani S, Tohgi M, Arai T, Ikeda K. Immunohistochemical study of the serotonergic neuronal system in an animal model of the mood disorder. Exp Neurol. 2006;201(1):60-65.
doi pubmed - Sanchez S, Sanchez C, Paredes SD, Cubero J, Rodriguez AB, Barriga C. Circadian variations of serotonin in plasma and different brain regions of rats. Mol Cell Biochem. 2008;317(1-2):105-111.
doi pubmed - Barassin S, Raison S, Saboureau M, Bienvenu C, Maitre M, Malan A, Pevet P. Circadian tryptophan hydroxylase levels and serotonin release in the suprachiasmatic nucleus of the rat. Eur J Neurosci. 2002;15(5):833-840.
doi pubmed - Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci. 1998;18(13):5045-5052.
pubmed - Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, et al. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8(12):e1000558.
doi pubmed - Hannibal J, Georg B, Fahrenkrug J. Differential expression of melanopsin mRNA and protein in Brown Norwegian rats. Exp Eye Res. 2013;106:55-63.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
