| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Letter to the Editor
Volume 10, Number 4, August 2020, pages 144-145
Rate, Clinical Features, Safety Profile and Outcome of Intravenous Thrombolysis for Acute Ischemic Stroke in Patients With Negative Brain Imaging
Ashkan Mowlaa, e, Haris Kamalb, Sandhya Mehlac, Peyman Shiranid, Robert N. Sawyerc
aDivision of Endovascular Neurosurgery, Department of Neurological Surgery, Keck School of Medicine, University of Southern California (USC), Los Angeles, CA, USA
bDepartment of Neurology, University of Texas Health Sciences Center at Houston, Houston, TX, USA
cDepartment of Neurology, University at Buffalo, State University of New York, Buffalo, NY, USA
dDepartments of Neurology and Neurosurgery, University of Cincinnati Medical Center, Cincinnati, OH, USA
eCorresponding Author: Ashkan Mowla, Division of Endovascular Neurosurgery, Department of Neurological Surgery, Keck School of Medicine, University of Southern California (USC), 1200 North State St., Suite 3300, Los Angeles, CA 90033, USA
Manuscript submitted May 17, 2020, accepted May 25, 2020, published online June 3, 2020
Short title: IV Thrombolysis for AIS
doi: https://doi.org/10.14740/jnr599
| To the Editor | ▴Top |
Intravenous (IV) recombinant tissue plasminogen activator (r-tPA) is the only Food and Drug Administration (FDA)-approved pharmacological therapy for acute ischemic stroke (AIS) [1-8]. The exigent time demand for IV r-tPA administration in AIS may not give the necessary time for thorough evaluation in patients presenting with AIS symptoms. As a result, IV thrombolysis of transient ischemic attack (TIA) or conditions mimicking stroke but with a subsequent different diagnosis might occur. In addition, IV r-tPA may sufficiently resolve ischemia such that subsequent brain diffusion-weighted magnetic resonance imaging (DWI-MRI) is negative in some patients. It would be expected that brain computed tomography (CT) scan and the more sensitive DWI-MRI would be negative in all these cases [9-11]. We sought to determine the rate, clinical characteristics, safety profile and outcome of brain imaging-negative patients treated with IV r-tPA for AIS in our large volume comprehensive stroke center, Buffalo General Hospital, Buffalo, NY, USA.
We retrospectively reviewed the medical records and brain imaging of patients who received IV r-tPA for AIS within 4.5 h of symptom onset in a 9.4-year period at our center. A subset of patients with absence of acute infarct/ischemia on their follow-up brain imaging were identified. The 24-h MRI-DWI was available for the majorly of the patients (90.1%). For those who had no follow-up brain MRI due to body size, claustrophobia, presence of metallic implants or foreign bodies, 24-h head CT was reviewed. We recorded age, admission National Institutes of Health Stroke Scale (NIHSS), discharge NIHSS, discharge modified Rankin Score (mRS), symptom onset to treatment time, clinical manifestations, discharge diagnosis and evidence of intracranial hemorrhage (ICH) on follow-up images for these patients.
A total of 637 patients received IV thrombolysis in our center during a 9.4-year period. Thirty-seven (5.8%) were found to have no evidence of acute ischemia/infarct on their follow-up imaging. DWI-MRI was available for 31 patients. Mean age was 65.2 ± 14, mean admission NIHSS was 8 and mean discharge NIHSS was 0. The most common symptom was left-sided hemiparesis in 45% followed by right-sided hemiparesis in 37% of those patients. Table 1 shows the clinical presentations of these 37 patients. The mean time of symptom onset to IV thrombolysis was 2.5 h. Twenty-two (59%) were diagnosed with TIA or averted stroke and the rest had non-vascular stroke mimics. The most common stroke mimics were migraine with aura, seizure disorder and psychogenic disorders (Fig. 1). No patient developed ICH on follow-up brain imaging or any IV r-tPA side effects such as angioedema or systemic bleeding. All patients were functionally independent on discharge, mRS 0 - 1.
 Click to view | Table 1. Clinical Presentations |
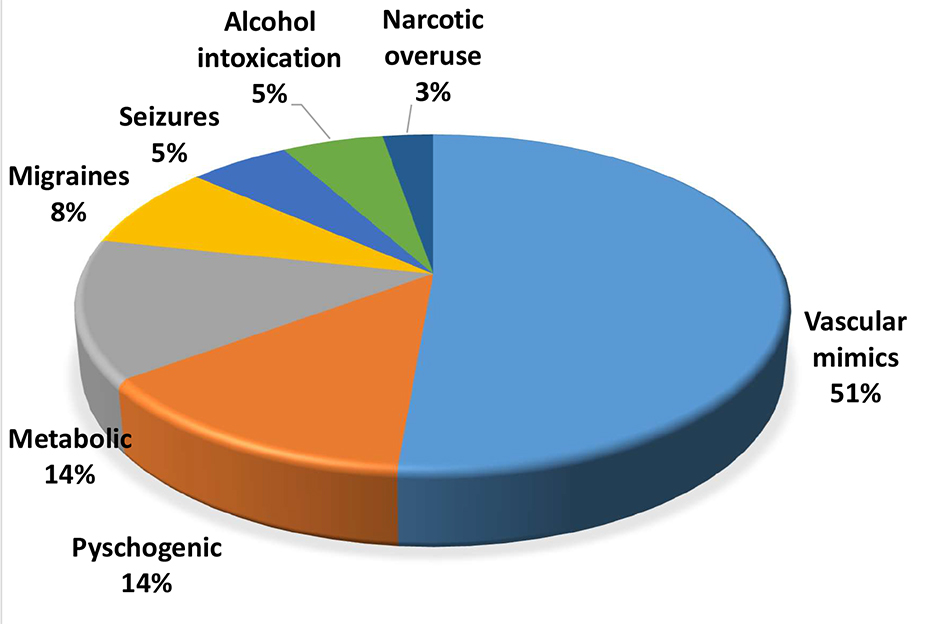 Click for large image | Figure 1. Etiologies of stroke mimics. |
IV thrombolysis is generally safe in patients with suspected AIS who have a follow-up negative brain imaging and delaying IV r-tPA administration in cases of doubt is not appropriate. Our results support the previous findings that the use of IV r-tPA appears to be safe in stroke mimics, and prognosis is generally favorable and complications are rather infrequent [9-11]. To the best of our knowledge, this is one of the largest reported series of patients with stroke mimics treated with IV r-tPA.
Acknowledgments
We would like to thank Ms. Deborah A. Steck, RN, MS, CNS, SCRN and Ms. Annemarie Crumlish, CCRC for their valuable and constructive guidance for our data collection.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
All authors have contributed to the concept, data collection, data analysis and writing of the article.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Mowla A, Doyle J, Lail NS, Rajabzadeh-Oghaz H, Deline C, Shirani P, Ching M, et al. Delays in door-to-needle time for acute ischemic stroke in the emergency department: A comprehensive stroke center experience. J Neurol Sci. 2017;376:102-105.
doi pubmed - Mowla A, Singh K, Mehla S, Ahmed MK, Shirani P, Kamal H, Krishna C, et al. Is acute reperfusion therapy safe in acute ischemic stroke patients who harbor unruptured intracranial aneurysm? Int J Stroke. 2015;10(Suppl A100):113-118.
doi pubmed - National Institute of Neurological Disorders. Stroke rt, P. A. Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587.
doi pubmed - Singh K, Mowla A, Mehla S, Ahmed MK, Shirani P, Zimmer WE, Sawyer RN, et al. Safety of intravenous thrombolysis for acute ischemic stroke in patients with preexisting intracranial neoplasms: a case series. Int J Stroke. 2015;10(3):E29-30.
doi pubmed - Mowla A, Kamal H, Lail NS, Vaughn C, Shirani P, Mehla S, Rajabzadeh-Oghaz H, et al. Intravenous thrombolysis for acute ischemic stroke in patients with thrombocytopenia. J Stroke Cerebrovasc Dis. 2017;26(7):1414-1418.
doi pubmed - Kamal H, Mowla A, Farooq S, Shirani P. Recurrent ischemic stroke can happen in stroke patients very early after intravenous thrombolysis. J Neurol Sci. 2015; 358(1-2):496-497.
doi pubmed - AbdelRazek MA, Mowla A, Hojnacki D, Zimmer W, Elsadek R, Abdelhamid N, Elsadek L, et al. Prior Asymptomatic Parenchymal Hemorrhage Does Not Increase the Risk for Intracranial Hemorrhage after Intravenous Thrombolysis. Cerebrovasc Dis. 2015; 40(5-6):201-204.
doi pubmed - Rajabzadeh-Oghaz H, Varble N, Davies JM, Mowla A, Shakir HJ, Sonig A, Shallwani H, et al. Computer-Assisted Adjuncts for Aneurysmal Morphologic Assessment: Toward More Precise and Accurate Approaches. Proc SPIE Int Soc Opt Eng. 2017;10134:101341C.
doi pubmed - Chernyshev OY, Martin-Schild S, Albright KC, Barreto A, Misra V, Acosta I, Grotta JC, et al. Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology. 2010;74(17):1340-1345.
doi pubmed - Spokoyny I, Raman R, Ernstrom K, Meyer BC, Hemmen TM. Imaging negative stroke: diagnoses and outcomes in intravenous tissue plasminogen activator-treated patients. J Stroke Cerebrovasc Dis. 2014;23(5):1046-1050.
doi pubmed - Guillan M, Alonso-Canovas A, Gonzalez-Valcarcel J, Garcia Barragan N, Garcia Caldentey J, Hernandez-Medrano I, Defelipe-Mimbrera A, et al. Stroke mimics treated with thrombolysis: further evidence on safety and distinctive clinical features. Cerebrovasc Dis. 2012;34(2):115-120.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
