| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Case Report
Volume 12, Number 3, October 2022, pages 132-138
Symptomatic Cerebral Vasospasm After Transsphenoidal Adenoma Resection of the Pituitary
Mehrdad Estakhra, Zahra Ghotbia, Afshin Borhani-Haghighia, Jason W. Tarpleyb, Reza Bavarsad Shahripourb, c, d
aClinical Neurology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
bSaint John’s Health Institute, Stroke Center, Santa Monica, CA, USA
cUCSD Comprehensive Stroke Center, Department of Neurosciences, University of California, San Diego, CA, USA
dCorresponding Author: Reza Bavarsad Shahripour, Providence Saint John’s Health Center, Neurovascular and Stroke Center, Santa Monica, CA 90404, USA
Manuscript submitted July 3, 2022, accepted August 30, 2022, published online October 22, 2022
Short title: Cerebral Vasospasm Post-Transsphenoidal Surgery
doi: https://doi.org/10.14740/jnr729
| Abstract | ▴Top |
Delayed cerebral ischemia (DCI) or vasospasm following transsphenoidal surgery (TSS) is a life-threatening and life-altering event that could potentially cause devastating complications, neurological morbidity, and high mortality. Herein, we report the case of a 16-year-old woman without a marked medical history and unusual complications after TSS for pituitary adenoma resection who developed cerebral vasospasm and infarction after TSS. To the best of our knowledge, this is the first case involving a patient under 18 years old and requiring thrombectomy after TSS. Additionally, we present our review of published case reports to underline the most often presentation characteristics, the interval between TSS and vasospasm, and therapeutic management. With respect to our case, we analyzed 27 cases of TSS complicated by symptomatic vasospasm. We include only pituitary adenoma resection and exclude other causes. The mean age was 47.33 ± 15.22 years at the time of surgery, and the male-to-female ratio was roughly equal among cases (female: 51.9%). Following surgery, 85.2% of patients experienced subarachnoid hemorrhage (SAH), and 22.3% experienced cerebrospinal fluid (CSF) leakage. The mean clinical presentation time of vasospasm ranged from 3 to 13 (mean: 7.5 ± 2.6) days after TSS. At discharge, 51.8% of cases at least had one neurologic complication, including six dead patients (18.5%). A high index of suspicion for vasospasm has been recommended because of the diverse symptoms of this rare condition and the high mortality rate.
Keywords: Transsphenoidal surgery; Stroke; Vasospasm; Transcranial Doppler; Adenoma
| Introduction | ▴Top |
Pituitary adenomas are among adults’ most frequently occurring primary intracranial tumors, with an estimated overall prevalence of 16.7% [1]. A common and well-tolerated procedure for removing pituitary adenomas is endoscopic endonasal surgery (EES). EES commonly encounters diabetes insipidus, epistaxis, hyposmia, and cerebrospinal fluid (CSF) leakage [2]. Cerebral vasospasm is a frequent possibility of subarachnoid hemorrhage (SAH) in around 67% of cases [3], with almost a third of them susceptible to disability and fatality [4, 5], despite advances in diagnostic and management techniques. Vasospasm’s mechanisms are unclear; extravascular clots, inherited factors, and dysfunctional autoregulation may play a role [6]. Beyond SAH, vasospasm has also been reported as a rare possible complication of cranial tumor resection [7, 8] following transsphenoidal surgery (TSS), particularly pituitary adenoma resection.
Herein, we present a 16-year-old female patient with a subsequent stroke resulting from cerebral vasospasm after pituitary adenoma resection who had not experienced other postoperative complications. In this case report, we argue that it is essential to maintain a constant threshold for acute vasospasm in patients that have undergone TSS. In addition, we summarize our review of published pituitary adenoma case reports to highlight the most common presentation characteristics, the interval between TSS and vasospasm, and the therapeutic management of these cases.
| Case Report | ▴Top |
Patient information and clinical findings
A 16-year-old female patient without marked past medical history presented with headache, left visual defect for about 2 months. The neurosurgery team evaluated her and magnetic resonance imaging (MRI) with T1, T2 with contrast confirmed a large heterogeneously enhancing mass centered within the sella extending into the suprasellar cistern measuring 2.1 × 3.3 × 3.3 cm favoring pituitary adenoma (Fig. 1). The intervention was planned with microscopic TSS. There was neither a CSF leak during the operation nor an SAH during or following TSS. The endocrinal postoperative evaluation showed no abnormal findings (thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), cortisone, growth hormone (GH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin (PRL)). After 3 days post-operation, the patient was discharged from the hospital. On a postoperative day 5 (POD) afternoon, she presented to the emergency room (ER) with acute right-sided weakness, numbness, facial droop, and headache (National Institutes of Health Stroke Scale (NIHSS) 8). She also complained of severe generalized throbbing headache (4/10 - 10/10) with nausea in the last 2 days before admission.
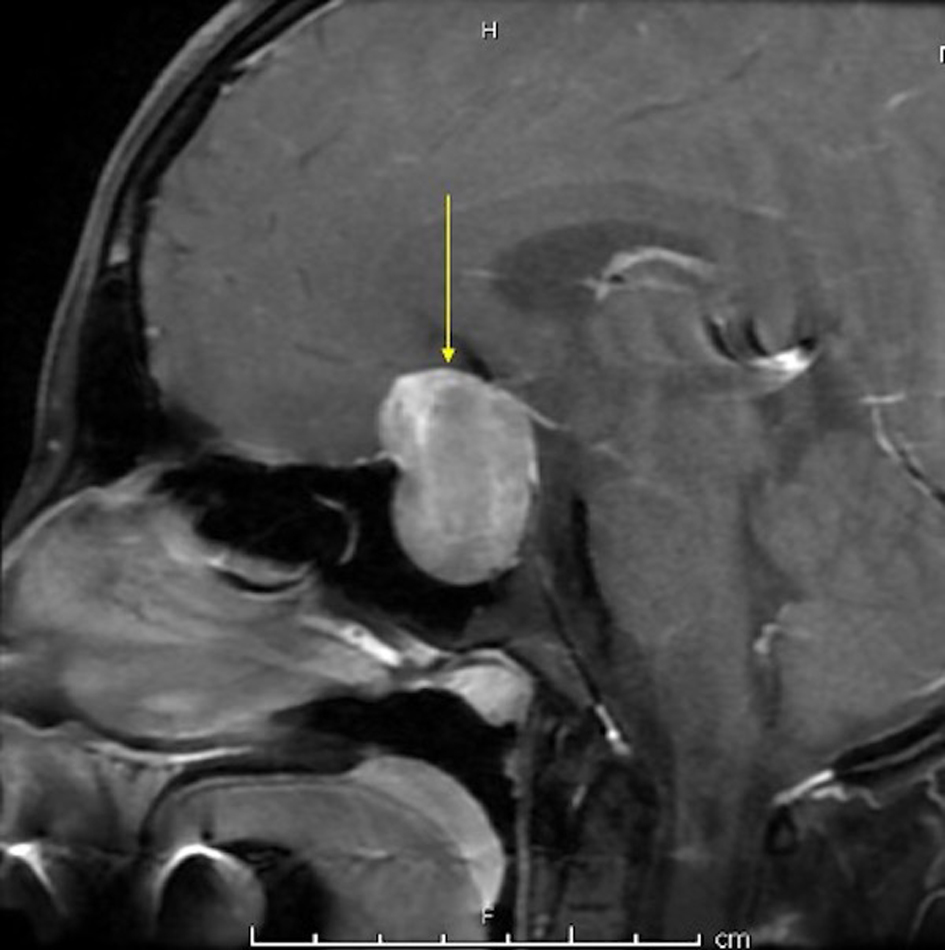 Click for large image | Figure 1. MRI with T1 with contrast showing a large heterogeneously enhancing mass centered within the sella extending into the suprasellar cistern measuring 2.1 × 3.3 × 3.3 cm in maximum dimensions. MRI: magnetic resonance imaging. |
Diagnostic assessment and therapeutic intervention
Due to new symptoms, she was evaluated through computed tomography (CT) of head and CT angiography (CTA) which revealed the occlusion of the left middle cerebral artery (MCA), M1 segment (Fig. 2a) with possible vasospasm in the proximal M1. Based on CT-ASPECTS and last known well, she underwent a successful thrombectomy (TICI3) (Fig. 2c). After transfer to the neuro-intensive care unit (NICU), all stroke and hyper-coagulopathy workups were done, and the results were unremarkable. Transthoracic echocardiogram (TTE) and transesophageal echo (TEE) did not show any abnormal findings. Transcranial Doppler (TCD) on the first day following thrombectomy revealed an increase in the mean blood flow velocity in the terminal internal carotid artery (t-ICA) (175 cm/s), M1 segment (200 m/s) and anterior cerebral artery, A1 segment (ACA) (180 cm/s) on the left side, which was consistent with severe vasospasm (Lindegaard ratio (LR): 8) (Fig. 3). During admission in the NICU, TCD findings confirmed the worsening of vasospasm in the left and right M1, left A1 and terminal ICA segments, with consistent cross-filling through the anterior communicating artery. On the first week of admission to the NICU, the patient underwent cerebral angiography and was treated locally with intra-arterial (IA) verapamil two times. Meanwhile, she experienced three episodes of deterioration with word-finding difficulty and worsening right-sided weakness (NIHSS increased from 3 to 8) with no significant change in CT perfusion (CTP). Her symptoms were very perfusion/blood pressure-dependent with dramatic response to elevated blood pressure on each episode. The patient was on high-dose vasopressors and nimodipine and her neurologic exam was stable (NIHSS: 2) as long as her blood pressure stayed above 120 mm Hg.
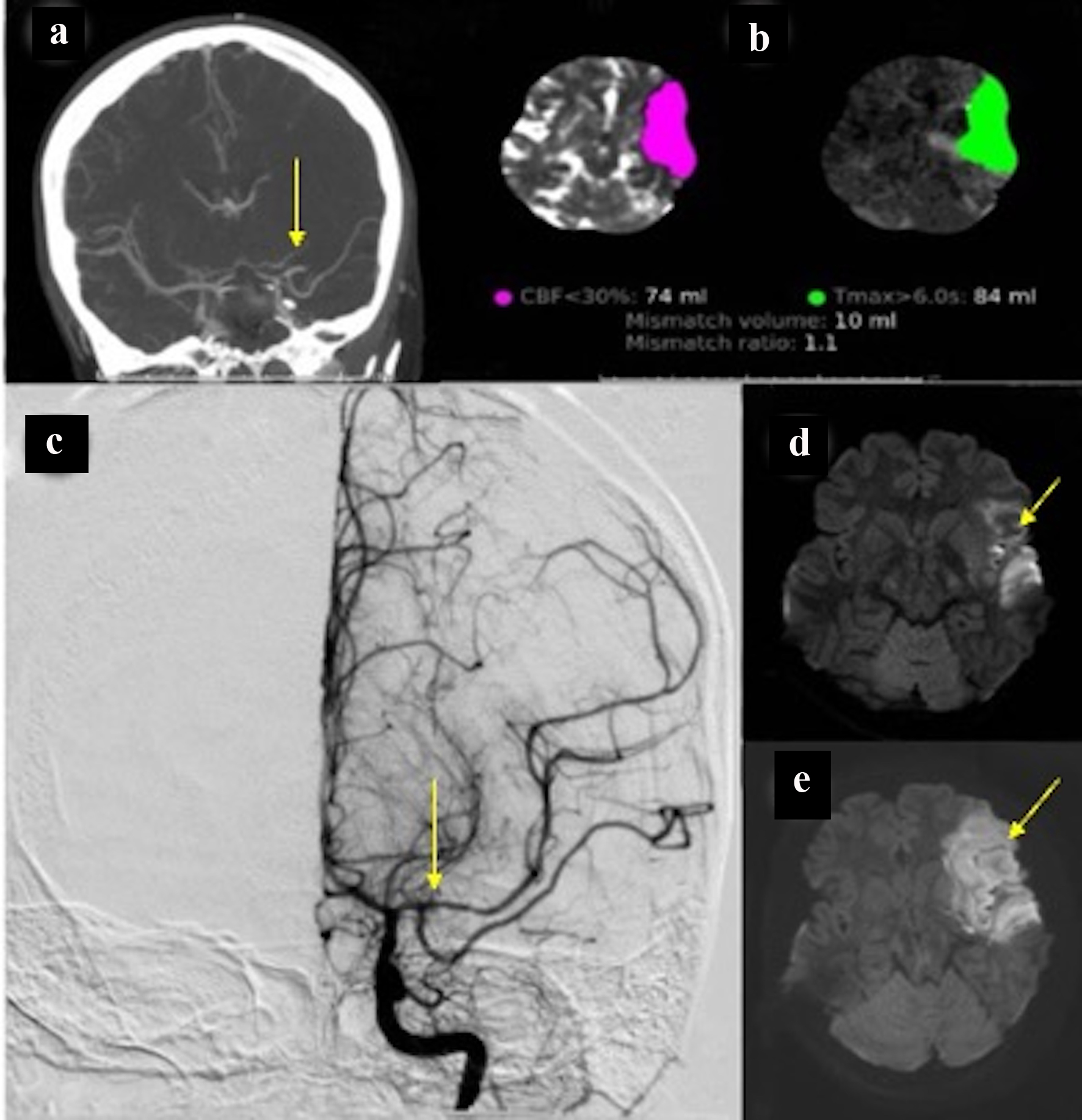 Click for large image | Figure 2. (a) CT angiography of head showing spasm in the proximal left M1 segment and middle M1 occlusion. (b) CT perfusion showing infarcted core in about 74 and 84 mL penumbra. (c) Cerebral angiography with successful recanalization. (d) Hyperintensity in the FLAIR sequence compatible with acute stroke. (e) Diffusion restriction in the left temporal lobe is consistent with acute stroke. CT: computed tomography. |
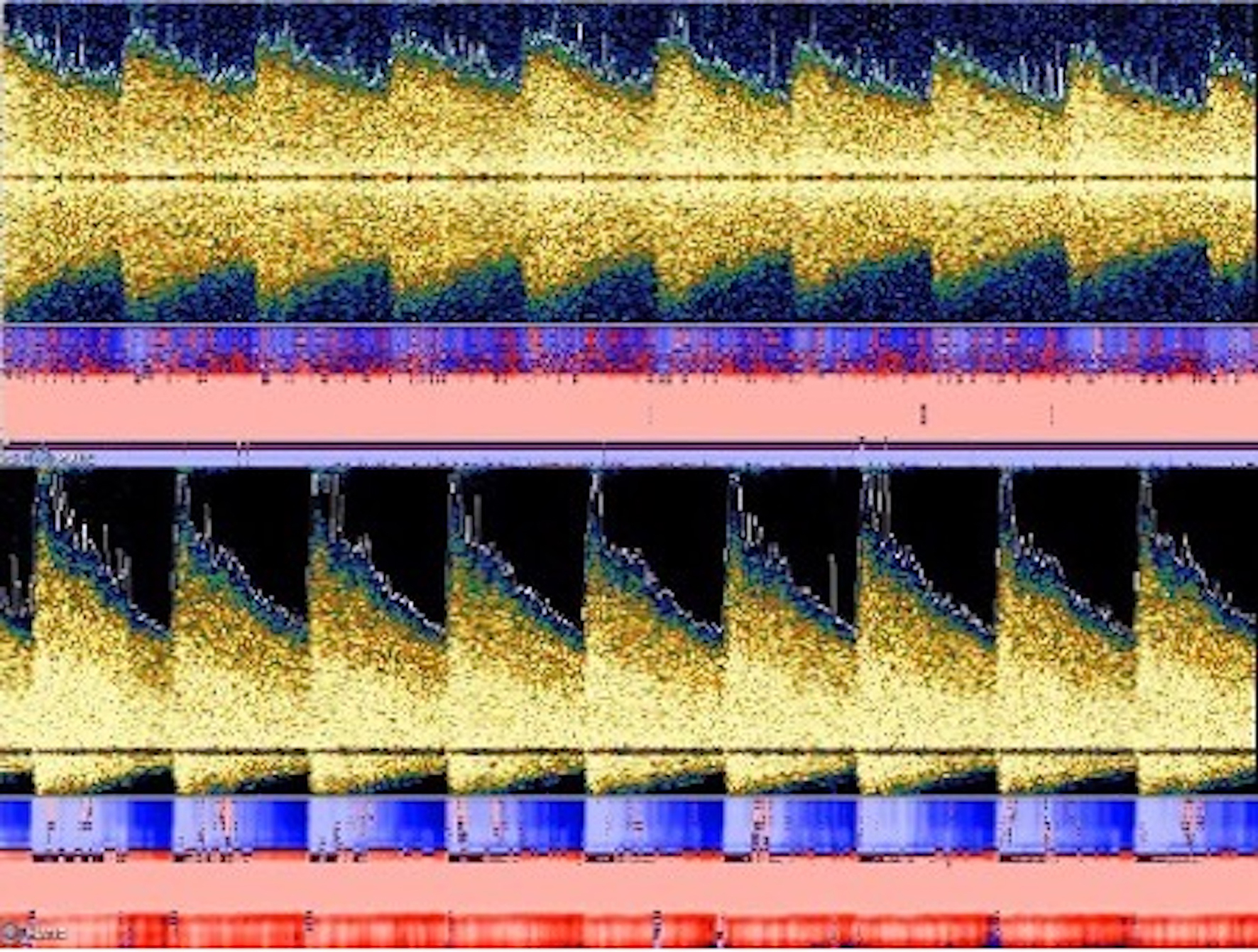 Click for large image | Figure 3. TCD findings showed increased mean flow velocity in both M1 and A1 segments on the left side with the Lindegaard ratio within the range of moderate to severe vasospasm. TCD: Transcranial Doppler. |
On POD 9, the patient became sudden onset aphasic, flaccid (1/5) on the right side and developed posturing on the right upper and lower limbs with a gaze to the left side (NIHSS: 22). The head CT perfusion showed a large perfusion deficit (Tmax > 6 s) in the left MCA territory (> 100 cc) with no significant core in cerebral blood flow (CBF). Given the confirmed vasospasm in the left ICA/MCA segments (indicated by cerebral angiography) and CTP findings, the neurosurgery team decided to perform hemi-craniotomy (HC) to prevent large MCA infarctions. It should be noted that throughout these episodes of deterioration, the patient did not experience any clinical seizure activity and the electroencephalogram (EEG) monitoring ruled out the epileptiform activity or electrographic seizure.
Follow-up and outcomes
HC was performed successfully, and in between, she was stable with mild dysarthria, facial droop and drifted in the left arm (NIHSS: 3). On the third week of admission, the mean flow velocity (MFV) and LR in A1 and M1 segments consistently improved on both sides. The first and second follow-up brain MRI revealed acute infarction in the left MCA distribution (Fig. 2d), and the last MRI on the fourth week did not show any new infarcted area. By POD 30, the patient was discharged to the rehabilitation center with NIHSS 2 (dysarthria and facial palsy).
Literature review
We searched the literature for all published cases of symptomatic vasospasm after TSS for sellar or suprasellar tumor resections. A search of Medline/PubMed and Google Scholar was conducted using the following search terms: “vasospasm” combined with “pituitary”, “transsphenoidal”, and “adenoma”. Medical subject headings (MeSH) are used to search PubMed. To find additional pertinent publications, we manually searched the references sections of the retrieved articles. This study only includes patients who have undergone pituitary adenoma resection and excludes patients who have undergone meningioma, craniopharyngioma, and other tumor resections. We also excluded articles published in languages other than English.
| Discussion | ▴Top |
Besides our patients, we also identified 27 cases of pituitary adenoma and 17 publications [6, 9-24] related to this condition. To the best of our knowledge, this is the first patient under 18 years old in our literature review who also has undergone a thrombectomy in her admission after uncomplicated TSS. Table 1 presents summarized data and analysis of these 27 cases.
 Click to view | Table 1. Clinical Characteristics of Reported Post-TSS Vasospasm Cases |
According to Table 1, the age ranged from 16 to 75 (mean: 47.33 ± 15.22) years at the surgery time, and the male-to-female ratio was roughly equal among cases (female: 51.9%). The POD after TSS ranged from 3 to 13 (mean: 7.5 ± 2.6) days. We have found that vasospasm occurs more frequently in anterior versus posterior circulations. Compared to the other arteries, ACA was the most involved artery (72%), and multiple territories vasospasm (ACA, MCA, and ICA) was reported in 36% of all cases. The data show that unilateral vasospasm has been detected more frequently than bilateral spasm (64% vs. 36%) (Table 1).
Aphasia, change in mental status, hemiparesis, and weakness are the common symptoms reported in patients with vasospasm (Table 1). A significant proportion of patients had SAH (85.2%) and CSF leakage (22.3%) after the TSS, and both have been noticed as possible etiology of vasospasm (Table 1). Meningitis is suspected as an underlying cause in a few cases [15]. Regarding outcome, 51.8% of patients at least suffer from minor neurological complications in discharge, including six dead patients (18.5%).
The location and vascular encasement are the leading players in terms of preoperative factors. Previous cases emerged that tumors located in the sella region with suprasellar extension are likely to develop vasospasm [6, 21-25]. Adjacency to Willis’ circle, particularly in vessel encasement or displacement, makes this location vulnerable. Vasospasm following surgery is significantly more likely to occur when there is vascular encasement or narrowing on preoperative imaging, regardless of the location of the tumor [26, 27].
Regarding post-TSS vasospasm risk factors, direct mechanical trauma to arteries [28], disturbed vascular tone [29], presence of SAH, hypothalamus sympathetic activation induced by manipulation [30, 31], meningitis, and irritation of vessels by excessive packing material [9, 15] have been recognized. Budnick et al reviewed the 34 cases of all-cause post-TSS vasospasm, including pituitary adenoma. They reported that SAH proceeded with the onset of symptomatic vasospasm in a large proportion (70.6%) of their patients [23]. Even though our patient did not suffer complications following surgery, including SAH or CSF leakage, our literature review found that 85.2% of those pituitary adenoma patients who experienced vasospasm post-TSS had SAH. Based on Kelly et al’s study [32] of 528 SAH patients with different etiologies, 58.5% of them experience cerebral vasospasm after the SAH. The clinical outcome of in-hospital mortality might be affected by SAH incidence. The study performed by Arboix et al on 184 SAH patients found that those who died within 72 h after symptoms onset had more progressive deficits, seizures, altered consciousness, limb weakness, sensory involvement, and basal ganglia hematoma than those without early death [33].
Regarding treatment, the approach to post-TSS vasospasm has been reported to be similar to post-SAH vasospasm. The treatment involves hyperdynamic treatments (hemodilution, hypertension, and hypervolemia) and intraarterial calcium channel blockers, such as verapamil and nimodipine. In our case, she had a dramatic response to vasopressor and elevated blood pressure, but in the long period after diagnosis (21 days). In our analysis, 18.5% of patients die from cerebral vasospasm following surgery for pituitary adenoma resection. Increasing awareness regarding post-TSS vasospasm may be helpful to diagnosis and treatment promptly and reduce mortality and morbidity in these cases.
This case report contains the following limitation: according to TCD findings, which confirmed prolonged extensive vasospasm in multiple territories (started from the first day after admission), in our opinion, the ischemic stroke resulted from severe spasms in the proximal M1 segment (which was shown in the first CTA on admission and confirmed in the follow-up cerebral angiography). Even so, we could not rule out the possibility that the patient’s ischemic stroke was caused by a thrombus secondary to another undetermined stroke etiologies in young adult.
To the best of our knowledge, this is the first case report on a patient under 18 years old who experienced cerebral vasospasm after uncomplicated TSS. It is crucial to consider cerebral vasospasms early post-procedure to provide effective treatment and improve clinical outcomes following delayed cerebral ischemia and vasospasm following TSS. Clinicians should raise awareness of this condition among their post-TSS patients, especially in pediatrics, as it manifests in several ways.
Learning points
To our knowledge, this is the only case of a patient under 18 years old requiring thrombectomy after TSS. Vasospasm and delayed cerebral ischemia are life-threatening outcomes that may result from TSS.
The diagnosis of cerebral vasospasm should be considered in individuals experiencing unexplained postoperative neurological symptoms even with an unremarkable brain CT scan.
The location, vascular encasement, and adjacency to the circle of Willis will significantly increase the risk of post-TSS vasospasm.
Acknowledgments
None to declare.
Financial Disclosure
We have no financial or funding disclosures.
Conflict of Interest
We have no financial or other conflict of interest.
Informed Consent
Informed consent to write and publish the case was obtained from the patient.
Author Contributions
ME provided the body of the case report and summarized the case. ZG provided the body of the case report and summarized the case. ABH drafted the discussion and revised the final draft. JWT revised the final draft and provided expertise on the clinical significance of the case. RBS drafted the discussion, edited the figures, participated in revising the final draft, and submitted the final paper for review.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. All data were extrapolated from referenced studies, and those studies may be reviewed for specific details of their data collection.
| References | ▴Top |
- Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613-619.
doi pubmed - Abbott J, Kirkby GR. Acute visual loss and pituitary apoplexy after surgery. BMJ. 2004;329(7459):218-219.
doi pubmed - Baggott CD, Aagaard-Kienitz B. Cerebral vasospasm. Neurosurg Clin N Am. 2014;25(3):497-528.
doi pubmed - Taki W, Sakai N, Suzuki H, PRESAT Group. Determinants of poor outcome after aneurysmal subarachnoid hemorrhage when both clipping and coiling are available: Prospective Registry of Subarachnoid Aneurysms Treatment (PRESAT) in Japan. World Neurosurg. 2011;76(5):437-445.
doi pubmed - Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31(6):1443-1451.
doi pubmed - Page PS, Kim DD, Hall GC, Koutourousiou M. Cerebral vasospasms following endoscopic endonasal surgery for pituitary adenoma resection in the absence of post-operative subarachnoid hemorrhage. J Neurol Stroke. 2016;4:00130.
doi - Ricarte IF, Funchal BF, Miranda Alves MA, Gomes DL, Valiente RA, Carvalho FA, Silva GS. Symptomatic cerebral vasospasm and delayed cerebral ischemia following transsphenoidal resection of a craniopharyngioma. J Stroke Cerebrovasc Dis. 2015;24(9):e271-273.
doi pubmed - Nash R, Elwell V, Brew S, Powell M, Grieve JP. Management strategy for treatment of vasospasm following transsphenoidal excision of craniopharyngioma. Acta Neurochir (Wien). 2016;158(11):2105-2108.
doi pubmed - Camp PE, Paxton HD, Buchan GC, Gahbauer H. Vasospasm after trans-sphenoidal hypophysectomy. Neurosurgery. 1980;7(4):382-386.
doi pubmed - Hyde-Rowan MD, Roessmann U, Brodkey JS. Vasospasm following transsphenoidal tumor removal associated with the arterial changes of oral contraception. Surg Neurol. 1983;20(2):120-124.
doi - Cervoni L, Salvati M, Santoro A. Vasospasm following tumor removal: report of 5 cases. Ital J Neurol Sci. 1996;17(4):291-294.
doi pubmed - Friedman JA, Meyer FB, Wetjen NM, Nichols DA. Balloon angioplasty to treat vasospasm after transsphenoidal surgery. Case illustration. J Neurosurg. 2001;95(2):353.
doi pubmed - Nishioka H, Ito H, Haraoka J. Cerebral vasospasm following transsphenoidal removal of a pituitary adenoma. Br J Neurosurg. 2001;15(1):44-47.
doi pubmed - Kasliwal MK, Srivastava R, Sinha S, Kale SS, Sharma BS. Vasospasm after transsphenoidal pituitary surgery: a case report and review of the literature. Neurol India. 2008;56(1):81-83.
doi pubmed - Popugaev KA, Savin IA, Lubnin AU, Goriachev AS, Kadashev BA, Kalinin PL, Pronin IN, et al. Unusual cause of cerebral vasospasm after pituitary surgery. Neurol Sci. 2011;32(4):673-680.
doi pubmed - Zada G, Du R, Laws ER, Jr. Defining the "edge of the envelope": patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. J Neurosurg. 2011;114(2):286-300.
doi pubmed - Puri AS, Zada G, Zarzour H, Laws E, Frerichs K. Cerebral vasospasm after transsphenoidal resection of pituitary macroadenomas: report of 3 cases and review of the literature. Neurosurgery. 2012;71(1 Suppl Operative):173-180; discussion 180-171.
doi pubmed - Kim EH, Oh MC, Kim SH. Angiographically documented cerebral vasospasm following transsphenoidal surgery for pituitary tumors. Pituitary. 2013;16(2):260-269.
doi pubmed - Mansouri A, Fallah A, Cusimano MD, Das S. Vasospasm post pituitary surgery: systematic review and 3 case presentations. Can J Neurol Sci. 2012;39(6):767-773.
doi pubmed - Bougaci N, Paquis P. Cerebral vasospasm after transsphenoidal surgery for pituitary adenoma: case report and review of the literature. Neurochirurgie. 2017;63(1):25-27.
doi pubmed - Osterhage K, Czorlich P, Burkhardt TR, Rotermund R, Grzyska U, Flitsch J. Symptomatic vasospasms as a life-threatening complication after transsphenoidal surgery. World Neurosurg. 2018;110:180-188.
doi pubmed - Suero Molina E, Di Somma A, Stummer W, Briganti F, Cavallo LM. Clinical vasospasm after an extended endoscopic endonasal approach for recurrent pituitary adenoma: illustrative case and systematic review of the literature. World Neurosurg. 2019;128:29-36.
doi pubmed - Budnick HC, Tomlinson S, Savage J, Cohen-Gadol A. Symptomatic cerebral vasospasm after transsphenoidal tumor resection: two case reports and systematic literature review. Cureus. 2020;12(5):e8171.
doi pubmed - Eseonu CI, ReFaey K, Geocadin RG, Quinones-Hinojosa A. Postoperative cerebral vasospasm following transsphenoidal pituitary adenoma surgery. World Neurosurg. 2016;92:7-14.
doi pubmed - Alotaibi NM, Lanzino G. Cerebral vasospasm following tumor resection. J Neurointerv Surg. 2013;5(5):413-418.
doi pubmed - Symon L. An experimental study of traumatic cerebral vascular spasm. J Neurol Neurosurg Psychiatry. 1967;30(6):497-505.
doi pubmed - Bejjani GK, Sekhar LN, Yost AM, Bank WO, Wright DC. Vasospasm after cranial base tumor resection: pathogenesis, diagnosis, and therapy. Surg Neurol. 1999;52(6):577-583; discussion 583-574.
doi - Laws ER, Jr. Vascular complications of transsphenoidal surgery. Pituitary. 1999;2(2):163-170.
doi pubmed - Chang SD, Yap OW, Adler JR, Jr. Symptomatic vasospasm after resection of a suprasellar pilocytic astrocytoma: case report and possible pathogenesis. Surg Neurol. 1999;51(5):521-526; discussion 526-527.
doi - Wilson JL, Feild JR. The production of intracranial vascular spasm by hypothalamic extract. J Neurosurg. 1974;40(4):473-479.
doi pubmed - Wilkins RH. Hypothalamic dysfunction and intracranial arterial spasms. Surg Neurol. 1975;4(5):472-480.
- Kelly PD, Yengo-Kahn AM, Tang AR, Jonathan SV, Reynolds RA, Ye F, Zhao Z, et al. Conditional vasospasm-free survival following aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2022;37(1):81-90.
doi pubmed - Arboix A, Marti-Vilalta JL. Predictive clinical factors of very early in-hospital mortality in subarachnoid hemorrhage. Clin Neurol Neurosurg. 1999;101(2):100-105.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
