| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Original Article
Volume 13, Number 1, September 2023, pages 43-49
The Clinical Spectrum of Multiple Sclerosis in a Tertiary Care Hospital
Komal Usha Chowdary Madinenia, c , Naveen Prasad S Vb, Vengamma Bhumab
aDepartment of Neurology, Mamatha Academy of Medical Sciences, Telangana 500090, India
bDepartment of Neurology, Sri Venkateswara Institute of Medical Sciences, Tirupati Andhra Pradesh 517507, India
cCorresponding Author: Komal Usha Chowdary Madineni, Department of Neurology, Mamatha Academy of Medical Sciences, Telangana 500090, India
Manuscript submitted February 11, 2023, accepted May 25, 2023, published online June 13, 2023
Short title: Clinical Spectrum of Multiple Sclerosis
doi: https://doi.org/10.14740/jnr750
| Abstract | ▴Top |
Background: Multiple sclerosis (MS) is a chronic autoimmune, inflammatory, demyelinating disease of the central nervous system (CNS) mediated by an inappropriate immune response within the body against the insulating myelin sheath.
Methods: Between January 2016 and March 2019, 20 diagnosed cases of MS were recruited for the study. Patient history was collected using a pre-designed standardized clinical proforma.
Results: The outcomes of the present study reveal a resemblance of MS patterns in Andhra Pradesh with India and with the West. MS is more common in women. The second and third decades are the most common. The incidence of the disease decreased with age. In comparison to relapsing-remitting MS (RRMS), primary-progressive MS (PPMS) had a younger onset age. The most common type of MS is RRMS. Individuals had different relapse rates. Relapses are more common in patients who first develop the disease at a young age. In PPMS patients, oligoclonal band (OCB) positivity is higher than in RRMS patients. A considerable number of individuals exhibited aberrant visual evoked potential (VEP) even in the absence of visual complaints. Disease-modifying drugs decreased the disease frequency and severity. Patients who started these drugs after 1 - 2 relapses had good results.
Conclusion: According to the findings, larger data sets are needed to completely characterize disease patterns. Given the rising prevalence of MS across India, it has become necessary to establish regional and national MS registries.
Keywords: Multiple sclerosis; Optic neuritis; Visual evoked potentials; Relapsing-remitting multiple sclerosis; Secondary-progressive multiple sclerosis; Primary-progressive multiple sclerosis
| Introduction | ▴Top |
Multiple sclerosis (MS), an autoimmune, demyelinating, and inflammatory central nervous system (CNS) disease, is caused by inappropriate immune responses within the body against the insulating myelin sheath. Although MS prevalence varies globally, it is high in European populations [1]. Few MS studies have been performed in some parts of India [2-4]. Recent studies [5-8] conducted in India have demonstrated increased MS prevalence across different Indian states owing to the growth of neurology as a subspecialty and the availability of modern diagnostic tools such as magnetic resonance imaging (MRI). This finding agrees with the 2013 Atlas of MS provided by the World Health Organization (WHO) and the Multiple Sclerosis International Federation.
There are four distinct subtypes of MS. About 85% of MS patients have the most prevalent kind, relapsing-remitting multiple sclerosis (RRMS) [9]. This kind of MS is defined by periods of sustained neurologic impairment interspersed with acute exacerbations. The hallmark of primary progressive is a subsequent decline in neurologic function without relapse [10]. Secondary progressive is characterized by an early relapsing-remitting phase that progresses to a persistent neurologic decline [10]. A more recent categorization, known as clinically isolated syndrome (CIS), describes a first clinical presentation that does not match the criteria for MS but may show focal or multifocal inflammatory demyelination [10]. Patients are more likely to experience a future flare that results in an MS diagnosis if they have a single neurologic episode that fits the criteria for CIS. The criteria depend mainly on the presence of dissemination in time and dissemination in space. Clinicians can now identify RRMS more quickly than before according to the 2017 McDonald diagnostic criteria. The 2017 update to the McDonald criteria requires the presence of MRI-identified lesions that are separated in both space and time for a diagnosis of RRMS, just like the 2010 version did. However, the new diagnostic criteria are now satisfied by the inclusion of cerebrospinal fluid (CSF) findings and more broadly localized lesions [11]. According to one study, diagnosis was made quicker in 63.5% of patients who used the 2017 McDonald criteria, taking 7.2 months less time than using the 2010 criteria.
There has been no research into the MS profile and prevalence in south India. This study aimed to evaluate the demographic and clinical profile of MS. The study also aimed to compare the VEPs, MRI of brain and spine, and oligoclonal bands (OCBs) among various patterns of MS.
| Materials and Methods | ▴Top |
Participants
This study recruited 20 patients who had been diagnosed with MS between January 2016 and March 2019 at a tertiary care referral center. The clinical diagnosis of MS was made using either the McDonald criteria 2010 or the revised McDonald criteria 2017, depending on the criteria available at the time of diagnosis. The inclusion criterion was a diagnosis of MS based on clinical and radiological evaluation, while the exclusion criteria were a family or previous history of other autoimmune diseases, not meeting McDonald’s diagnostic criteria, or testing positive for aquaporin-4 antibodies or anti-myelin oligodendrocyte glycoprotein (MOG) antibodies. IRB is not taken as the information is primarily collected from the medical records department. The study was conducted in compliance with the ethical standards of the institution on the human subjects as well as with Helsinki Declaration.
Data collection
A pre-designed standardized questionnaire in the form of a proforma was prepared to collect patient history. Demographic, clinical, biochemical, radiological, and electrophysiological details were recorded for patients whose information was available by reviewing their medical files. The demographic and clinical data collected included age, gender, religion, region, socio-economic status, disease duration, signs and symptoms, disease course (relapsing-remitting, secondary-progressive, and primary-progressive), number of attacks, family history of the disease, and mode of treatment. Relevant investigations were conducted to diagnose MS and exclude other conditions that mimic MS. Serum samples were collected from all patients to test for aquaporin-4 antibodies or anti-MOG antibodies. CSF was collected to examine OCBs. VEPs, brainstem auditory evoked responses, and somatosensory evoked potentials were studied in all study participants. MRI MS protocol was conducted on all patients, which included MRI of the brain and the entire spine, both with plain and contrast sequences.
Statistical analysis
The demographic and clinical variables were presented as frequencies and percentages. For age at the time of analysis, disease duration, number of attacks, and number of days in the hospital, the mean and standard deviation (SD) were used to describe the data.
| Results | ▴Top |
All 20 participants recruited for the study belong to South India. Female participants outnumbered male participants with a female-to-male ratio of 4:1. The incidence of MS inversely decreased with age. The mean age at MS onset was 26 years in females and 28 years in males (range: 10 to 52 years). Most patients were in their third decade at disease onset. Table 1 presents the distribution of various clinical characteristics and demographic parameters.
 Click to view | Table 1. Demographic and Clinical Features of Study Subjects |
RRMS, primary-progressive MS (PPMS), and secondary-progressive MS (SPMS) occurred in 80%, 15%, and 5% of cases, respectively (Fig. 1). The mean age at onset of PPMS was 16 years, which was lower than that of RRMS onset at 28 years. The SD of the mean onset age was lower in PPMS than in RRMS (range: 10 - 22 and 10 - 52 years, respectively). Only one patient had SPMS during the time of analysis. Optic neuritis was the most common initial presentation (30% of cases), followed by pyramidal involvement (25% of cases), cerebellar symptoms (20% of cases), brain stem symptoms (15% of cases), sensory symptoms (5% of cases), and isolated facial palsy (5% of cases). Six patients (30%) had bladder involvement; of them, two had early bladder involvement and four had late bladder involvement. One of the patients with brain stem involvement presented with internuclear ophthalmoplegia (INO). The mean number of attacks was 2.85 ± 2.6. Four of the 16 RRMS patients experienced a single relapse, and the total disease duration was less than 1 year. Five of them had two relapses in a disease duration of 1 year in three patients, less than 5 years in one patient, and more than 5 years in one patient. Moreover, the mean, longest, and shortest disease durations were 5 ± 6.1, 20 years, and 1 week, respectively. Investigations, including hemogram and biochemical parameters, were normal. Furthermore, a CSF study revealed OCB positivity in 40% of cases. Moreover, 31% of RRMS patients and 67% of PPMS patients had OCBs (Fig. 1). Visual symptoms appeared in six (30%) patients. Among them, abnormal VEP was recorded in five (25%) cases. VEP was abnormal even without any visual symptoms in 11 patients. All PPMS patients exhibited abnormal VEPs without visual symptoms (Fig. 2). MRI findings were suggestive of MS in all cases. MRI of cervical and dorsal spine areas was abnormal and also compatible with MS in 50% of cases. Table 2 lists MRI findings at the first visit (first MRI) to the hospital and after the first relapse (second MRI). The total lesion number was determined. Eleven and sixteen patients demonstrated infratentorial lesions at the first and second MRI, respectively.
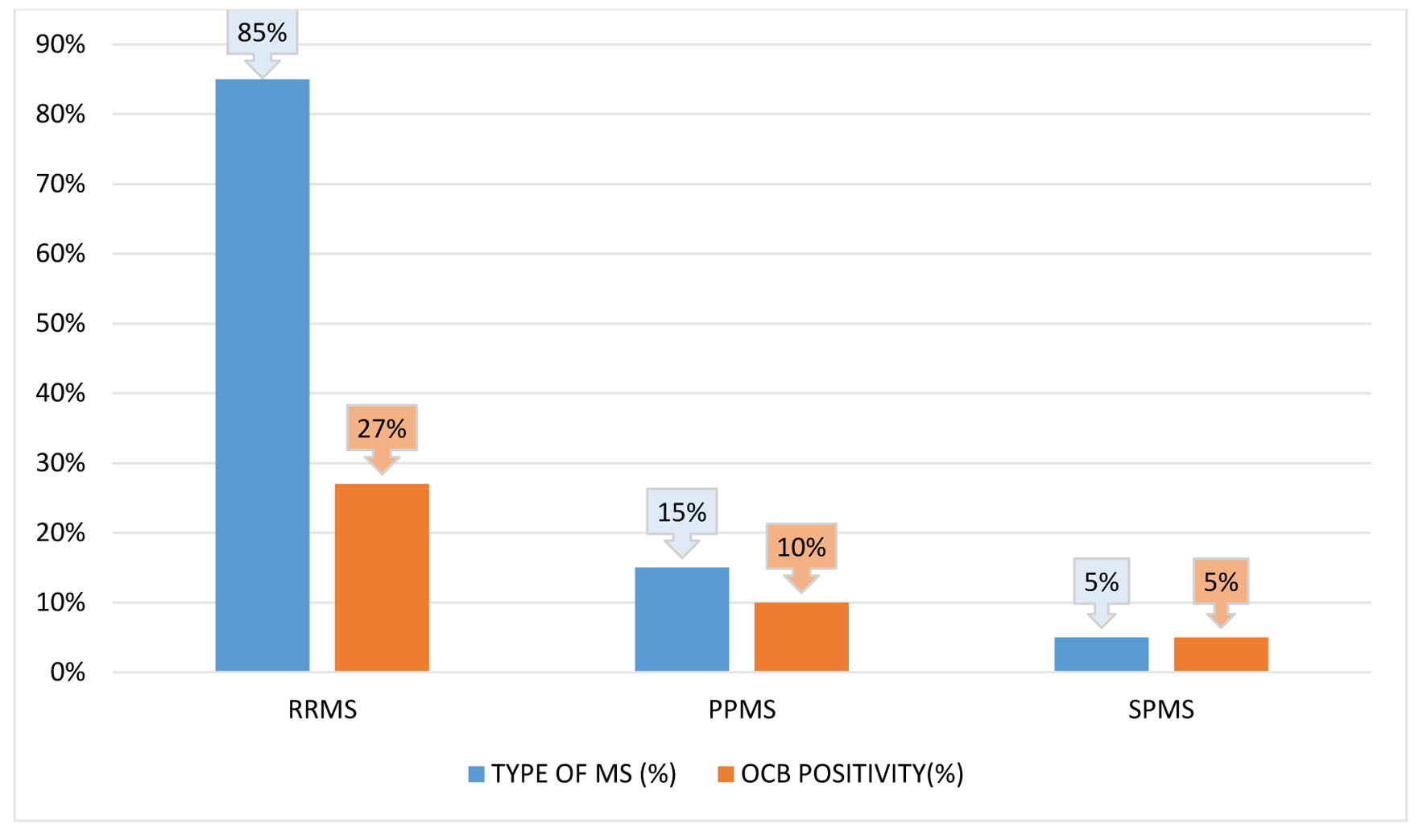 Click for large image | Figure 1. Relapsing-remitting multiple sclerosis (RRMS), secondary-progressive multiple sclerosis (SPMS), and primary-progressive multiple sclerosis (PPMS) are each represented in a bar diagram with respect to the relative frequency of oligoclonal bands (OCBs) positive status. |
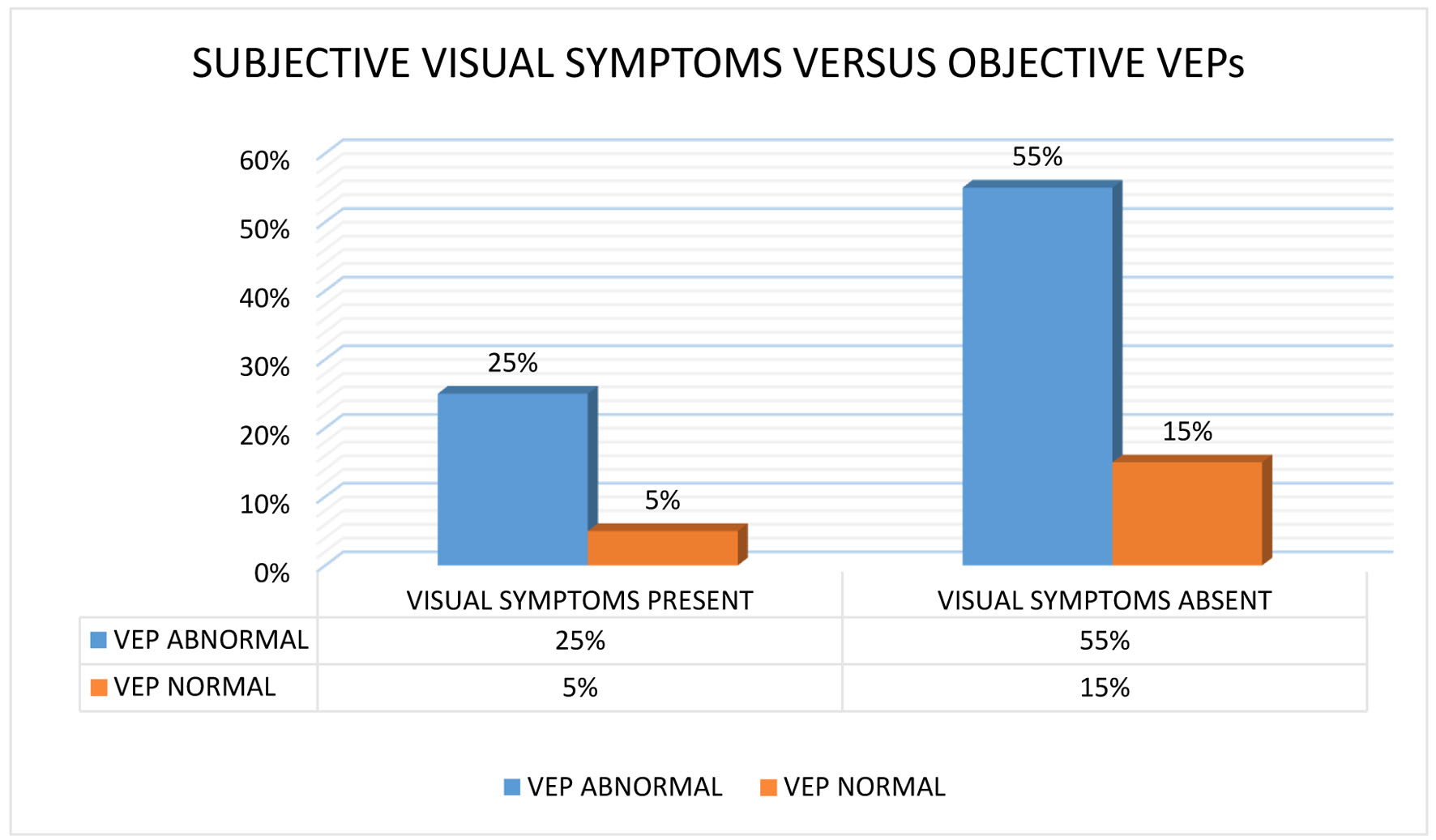 Click for large image | Figure 2. A bar graph comparing the proportional frequency of the visual evoked potentials (VEPs) positive status to the frequency of positive clinical visual symptoms. |
 Click to view | Table 2. MRI Characteristics |
All patients underwent symptomatic and steroid (methylprednisolone) treatment (100%) and also received one of the disease-modifying drugs (100%): azathioprine (40%), interferon beta-1a (30%), teriflunomide (15%), rituximab (5%), methotrexate (5%), and cyclophosphamide (5%). One patient on azathioprine developed leukopenia (5%), requiring its discontinuation. Two out of eight patients who received long-term oral corticosteroids developed steroid-induced side effects such as Cushing’s syndrome and hypertension. On average, the patients stayed in the hospital for 21.3 ± 17 days.
| Discussion | ▴Top |
This study investigated MS patterns in Asian populations. Data on MS from South India are inadequate. The female-to-male ratio of 4:1 suggested a higher female predominance, which is similar to the results of previous studies from India and other countries [4, 11-15]. Fourteen (70%) patients were adults, and the remaining six patients (30%) were adolescents at illness onset. The maximum number of cases had disease onset in their 20s, indicating a higher predisposition in young adults. New case numbers decreased with an increase in age [16]. The mean MS onset age was 27 ± 12 years (range: 10 - 52 years) [16]. PPMS patients had an earlier MS onset (mean age: 16 years) than SPMS and RRMS patients. The most common mode of disease course was RRMS (80%). The number of relapses increased with disease duration. The mean duration of the disease was 5 years, with the longest being 20 years. Most clinical parameters were like those reported in other parts of India and the West. The most predominant symptoms were visual, pyramidal (Fig. 3), sensory disturbances (Fig. 4), and cerebellar symptoms [17]. Two patients had cranial neuropathy, and six had bladder involvement (early and late in two and four patients, respectively). A single patient had INO at presentation. The presence of OCBs was observed in CSF samples of 40% of patients; 31% of RRMS cases and 60% of PPMS cases had OCB positivity. This agrees with reports from different Indian states that indicated fewer OCB-positive cases [18, 19]. This is less than the rates reported for Caucasians [20, 21], but it is comparable to those found in southern Chinese and Japanese populations. The varying seroprevalence of CSF-OCB may be influenced by the population under observation and different study designs [22]. Most other studies agree that the clinical characteristics of the OCB-positive and OCB-negative groups were comparable [23]. The OCB positivity rate is higher in PPMS cases compared with RRMS cases [24].
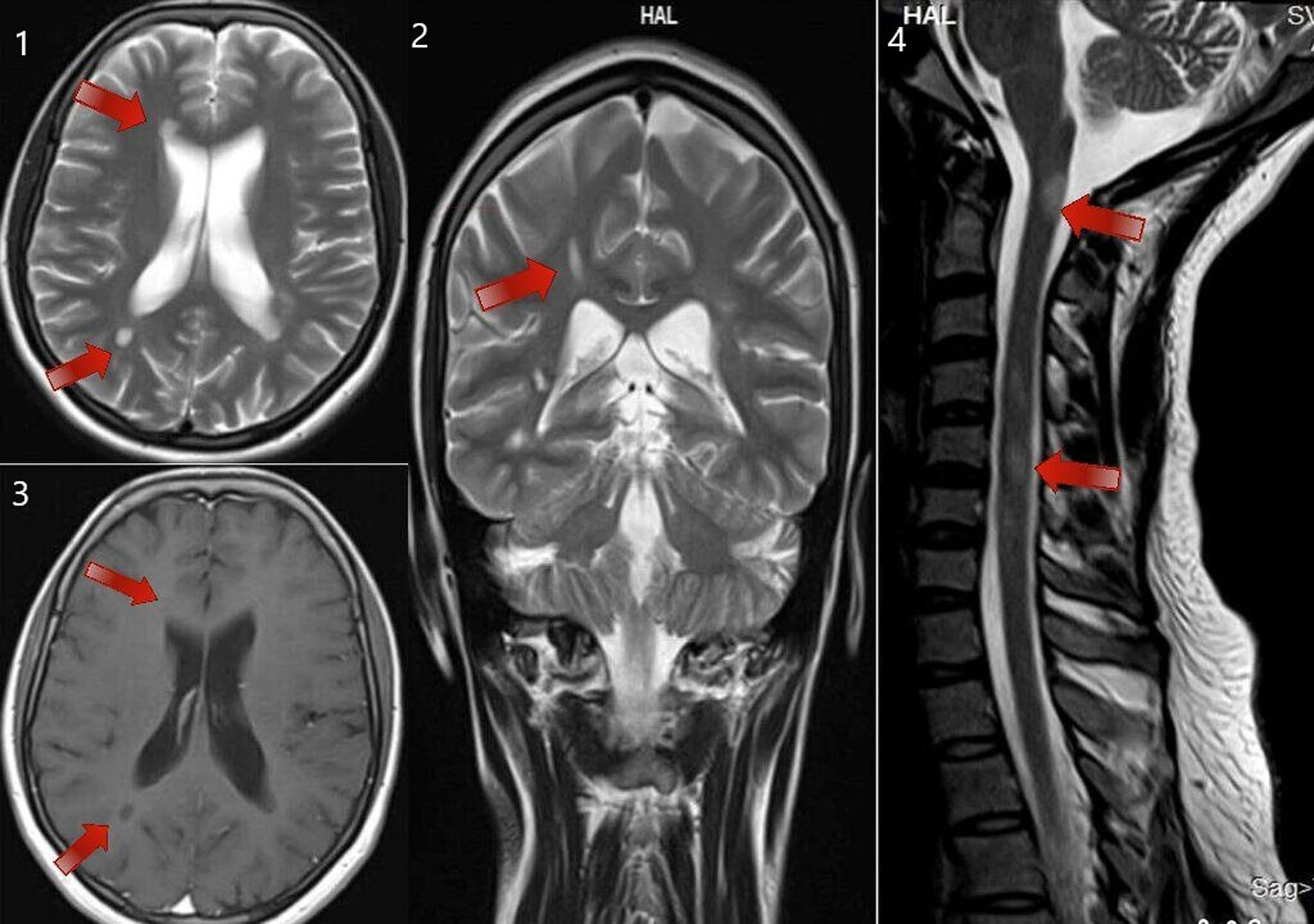 Click for large image | Figure 3. MRI of brain T2 sequence axial section showing periventricular deep white matter lesions (as shown by the arrows in the sequence labeled as 1). MRI of brain T2 sequence coronal section in the corresponding regions shows an abnormal hyperintense signal (as shown by the arrows in the sequence labeled as 2). MRI of brain post-contrast T1 sequence in the corresponding regions shows a hypointense signal (as shown by the arrows in the sequence labeled as 3). MRI of the cervical spine sagittal section shows short segment abnormal cord hyperintensity (as shown by the arrows in the sequence labeled as 4). These images correspond to a 24-year-old woman who presented with right-hand monoplegia. She also had a recent history of sudden onset decreased vision in the right eye which has subsided spontaneously. MRI: magnetic resonance imaging. |
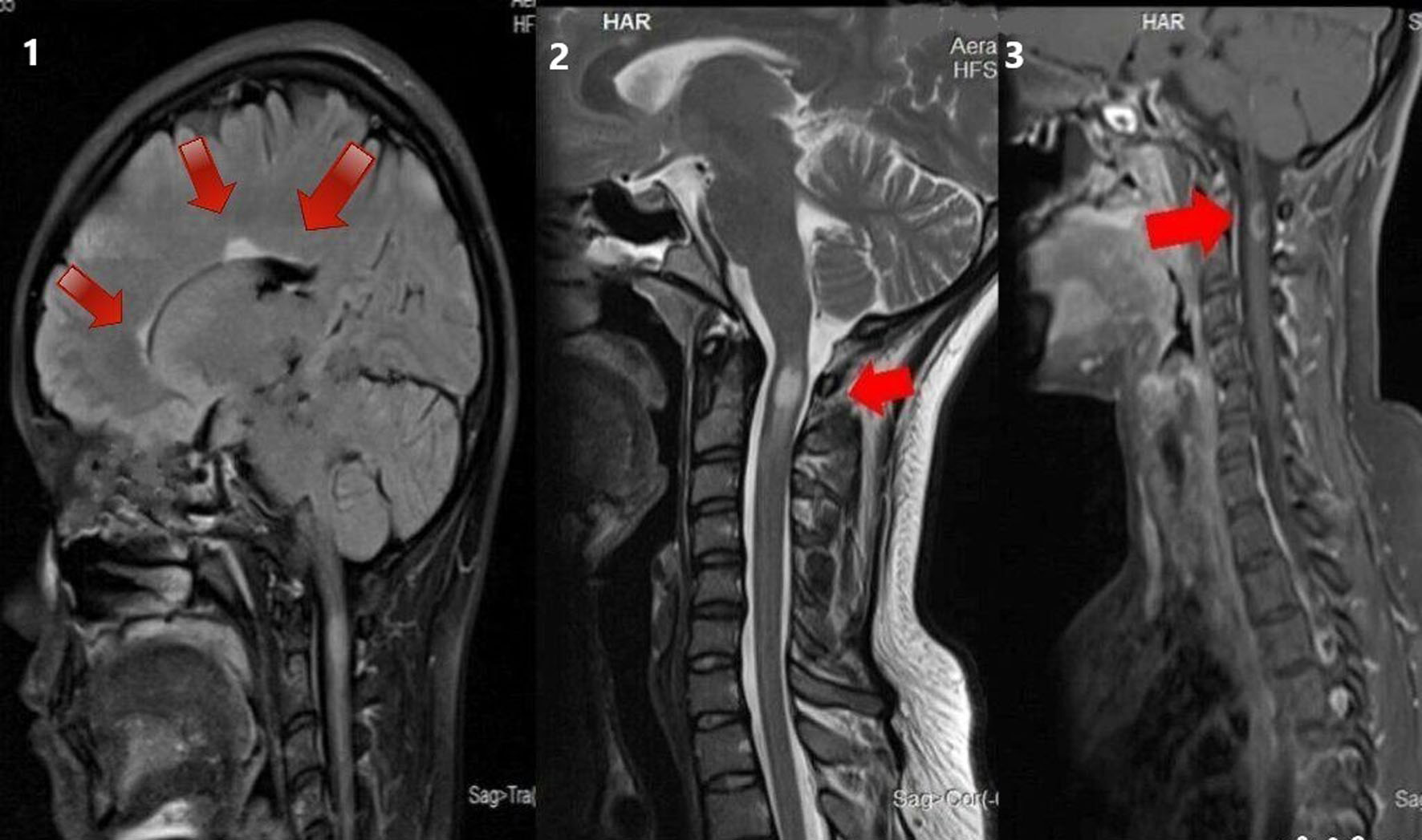 Click for large image | Figure 4. MRI of brain T2 sagittal sequence showing three periventricular lesions (as shown by the arrows in the sequence labeled 1). MRI of cervical spine T2 sagittal sequence showing short segment hyperintensity (as shown by the arrows in the sequence labeled 2). MRI of cervical spine T1 post-contrast sagittal sequence showing contrast enhancement in the corresponding region (as shown by arrows in the sequence labeled 3) in a 28-year-old woman with a 10-day history of pain and paraesthesia in the occipital and neck region. MRI: magnetic resonance imaging. |
The patients did not have any significant family history of MS. These findings agree with those reported in another Asian study [25]. All patients underwent symptomatic and steroid (methylprednisolone) treatment (100%) and received one of the disease-modifying drugs among azathioprine, Avonex (interferon beta-1a), teriflunomide, rituximab, cyclophosphamide, and methotrexate. Two patients developed a recurrence even after receiving treatment with a first-line disease-modifying therapy (DMT) and were switched to receive second-line DMTs. PPMS patients exhibited the longest hospital stay (24 days). We are the first to explore MS patterns in South India. The slight difference among certain MS parameters may be due to multifarious genetic and local environmental elements in MS etiology. The relapse frequency varied among individuals. A considerable proportion demonstrated abnormal VEPs without visual symptoms. Disease-modifying agents reduced disease frequency and severity. Patients who received these agents after 1 to 2 relapses exhibited satisfactory outcomes with disease remission. The results of this study suggest that disease-modifying agents can effectively reduce the frequency and severity of MS relapses. However, some patients may still experience relapses while using immunomodulation and require a change in medication. Repeat MRI scans in patients receiving immunomodulation showed fewer new asymptomatic lesions, indicating the effectiveness of these treatments in controlling the progression of the disease. Additionally, the use of the 2017 McDonald criteria allowed for the early diagnosis of MS in patients who would have previously been classified as having CIS under the 2010 criteria. These findings highlight the importance of early and accurate diagnosis of MS and the potential benefits of disease-modifying agents in managing the disease.
Conclusion
This study sheds light on the patterns of MS in the South Indian population, with a higher female predominance and a common RRMS disease course. The study findings suggest the need for larger and more comprehensive studies to better understand MS patterns in the region, including the establishment of a regional and national MS registry in India. Future studies should also investigate environmental factors and other potential risk factors in MS etiology. Overall, this study provides a foundation for further research and potential interventions to address the increasing prevalence of MS in South India.
Acknowledgments
This original research has been presented in “APNEUROCON 2019” conducted at Vishakhapatnam, Andhra Pradesh, India.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Komal Usha Madineni: collection of data, manuscript writing, revision, statistical analysis. Naveen Prasad S V: collection of data, research design, revision. Vengamma Bhuma: research design, revision.
Data Availability
The data supporting the findings are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502-1517.
doi pubmed - Bhatia M, Behari M, Ahuja GK. Multiple sclerosis in India: A.I.I.M.S. experience. J Assoc Physicians India. 1996;44(11):765-767.
pubmed - Pandit L, Subramanya R, Rao SN. Multiple sclerosis in coastal Karnataka. Neurol India. 1993;41(3):143-146.
pubmed - Bhatia R, Bali P, Chaudhari RM. Epidemiology and genetic aspects of multiple sclerosis in India. Ann Indian Acad Neurol. 2015;18(Suppl 1):S6-S10.
doi pubmed pmc - Pandit L, Kundapur R. Prevalence and patterns of demyelinating central nervous system disorders in urban Mangalore, South India. Mult Scler. 2014;20(12):1651-1653.
doi pubmed - Singhal BS, Wadia NH. Profile of multiple sclerosis in the bombay region. On the basis of critical clinical appraisal. J Neurol Sci. 1975;26(2):259-270.
doi pubmed - Zahoor I, Haq E. Multiple sclerosis in India: Iceberg or volcano. J Neuroimmunol. 2017;307:27-30.
doi pubmed - Kumar S, Rohatgi A, Chaudhari H, Thakor P. Evolving Landscape of Multiple Sclerosis in India: Challenges in the Management. Ann Indian Acad Neurol. 2018;21(2):107-115.
doi pubmed pmc - Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389(10076):1336-1346.
doi pubmed - Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
doi pubmed pmc - Trapp BD, Vignos M, Dudman J, Chang A, Fisher E, Staugaitis SM, Battapady H, et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol. 2018;17(10):870-884.
doi pubmed pmc - Khadilkar S, Sahni AO, Agarwal SA. A case-control study of environmental risk factors in Indians with multiple sclerosis. Neurol Asia. 2005;10:47-52
- Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9(5):A387-394.
doi pubmed - Zahoor I, Asimi R, Haq E. No evidence for a role of Ile587Val polymorphism of EIF2B5 gene in multiple sclerosis in Kashmir Valley of India. J Neurol Sci. 2015;359(1-2):172-176.
doi pubmed - Shull C, Hoyle B, Iannotta C, Fletcher E, Curan M, Cipollone V. A current understanding of multiple sclerosis. JAAPA. 2020;33(2):19-23.
doi pubmed - Cheraghmakani H, Baghbanian SM, HabibiSaravi R, Azar A, Ghasemihamedani F. Age and sex-adjusted incidence and yearly prevalence of multiple sclerosis (MS) in Mazandaran province, Iran: An 11-years study. PLoS One. 2020;15(7):e0235562.
doi pubmed pmc - Ford H. Clinical presentation and diagnosis of multiple sclerosis. Clin Med (Lond). 2020;20(4):380-383.
doi pubmed pmc - Jena SS, Alexander M, Aaron S, Mathew V, Thomas MM, Patil AK, Sivadasan A, et al. Natural history of multiple sclerosis from the Indian perspective: Experience from a tertiary care hospital. Neurol India. 2015;63(6):866-873.
doi pubmed - Syal P, Prabhakar S, Thussu A, Sehgal S, Khandelwal N. Clinical profile of multiple sclerosis in north-west India. Neurol India. 1999;47(1):12-17.
pubmed - Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol. 2003;2(2):117-127.
doi pubmed - Lu T, Zhao L, Sun X, Au C, Huang Y, Yang Y, Bao J, et al. Comparison of multiple sclerosis patients with and without oligoclonal IgG bands in South China. J Clin Neurosci. 2019;66:51-55.
doi pubmed - Petzold A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J Neuroimmunol. 2013;262(1-2):1-10.
doi pubmed - Anagnostouli M, Christidi F, Zalonis I, Nikolaou C, Lyrakos D, Triantafyllou N, Evdokimidis I, et al. Clinical and cognitive implications of cerebrospinal fluid oligoclonal bands in multiple sclerosis patients. Neurol Sci. 2015;36(11):2053-2060.
doi pubmed - Karrenbauer VD, Bedri SK, Hillert J, Manouchehrinia A. Cerebrospinal fluid oligoclonal immunoglobulin gamma bands and long-term disability progression in multiple sclerosis: a retrospective cohort study. Sci Rep. 2021;11(1):14987.
doi pubmed pmc - Wasay M, Khatri IA, Khealani B, Sheerani M. MS in Asian countries. Int MS J. 2006;13(2):58-65.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
