| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Original Article
Volume 14, Number 2, July 2024, pages 59-67
Tracheostomy and Gastrostomy After Decompressive Craniectomy: What Surrogates Need to Know
Aidan J. Jacobsena, Chase Schlesselmanb, Norman Scott Litofskya, c
aDepartment of Neurological Surgery, University of Missouri School of Medicine, Columbia, MO, USA
bOffice of Medical Research, University of Missouri School of Medicine, Columbia, MO, USA
cCorresponding Author: Norman Scott Litofsky, Department of Neurological Surgery, University of Missouri-Columbia School of Medicine, Columbia, MO 65212, USA
Manuscript submitted February 29, 2024, accepted June 18, 2024, published online July 5, 2024
Short title: Tracheostomy After Decompressive Craniectomy
doi: https://doi.org/10.14740/jnr779
| Abstract | ▴Top |
Background: Many patients require tracheostomy or feeding gastrostomy for airway stability and proper nutrition after decompressive craniectomy (DC) for trauma or stroke. Tracheostomy and/or gastrostomy implementation may impact decisions for end-of-life care. The authors hypothesized that patients surviving DC were more likely to have received a tracheostomy and/or gastrostomy than those who did not survive. Furthermore, the authors hypothesized that patients who did not receive a tracheostomy and/or gastrostomy were more likely to proceed to withdrawal of life-preserving care as the alternative.
Methods: Data collected from DC patients from 2014 to 2022 included age, admission setting, diagnosis (stroke, trauma), admission Glasgow Coma Scale (GCS), preoperative GCS, time to decompression after presentation, and socioeconomic factors. Patients with tracheostomy and/or gastrostomy were compared to patients not receiving tracheostomy or gastrostomy for the above characteristics and their outcomes (discharge disposition, Glasgow Outcome Scale (GOS), modified Rankin Scale (mRS), status of inpatient hospice/palliative care, and cause of death). Statistical tests used for analysis included Chi-square, two-sided t-test, and multiple logistic regression models (significance < 0.05).
Results: Sixty-six patients were included. More patients without tracheostomy and/or gastrostomy (32 patients) died than patients who received tracheostomy and/or gastrostomy (34 patients) (P = 0.0394). GOS and mRS did not differ between patients with tracheostomy and/or gastrostomy and patients without tracheostomy or gastrostomy (P = 0.1331 and 0.5421, respectively). Patients without tracheostomy or gastrostomy were more likely to have been placed on general inpatient hospice (GIP) (P = 0.0183) or had comfort care initiated (P = 0.00913).
Conclusions: Patients who survive after DC are more likely to have received tracheostomy and/or gastrostomy than those who did not survive. Patients who seek end-of-life care, including withdrawal of care and GIP, are more likely to not receive tracheostomy or gastrostomy.
Keywords: Decompressive craniectomy; Tracheostomy; Hemicraniectomy; Glasgow Outcome Score; Palliative care
| Introduction | ▴Top |
Decompressive craniectomy (DC) can be a life-saving measure for patients with refractory elevated intracranial pressure (ICP) resulting from trauma or stroke. Early DC can lead to better patient outcomes and decreased mortality [1]. Despite DC, recovery can be strenuous, and many patients require tracheostomy and/or gastrostomy); between 15% and 45% of stroke patients require tracheostomy [2], and between 16% and 24% of severe traumatic brain injury (TBI) patients receive gastrostomy [3]. These two procedures can be performed at the same time or separately, but they are often considered together as critical care procedures in severely ill patients [3]. Their use following surgery can decrease mortality and facilitate functional recovery in such patients with potential return to baseline metrics [4, 5].
While beneficial, tracheostomy and gastrostomy are both invasive procedures that can influence patient goals. Tracheostomy and/or gastrostomy can create functional, physical, and psychosocial challenges for patients [6, 7], including higher rates of depression and withdrawal of active participation in their medical care [8]. Additionally, palliative care length of stay (LOS) for stroke patients with tracheostomy is higher than those without [9]. These impacts warrant consideration during the progression of care for patients who may require DC, particularly in addressing end-of-life care and optimization of resource utilization [10].
DC patients and families may not understand the post-surgery likelihood of receiving a tracheostomy or gastrostomy. The addition of a tracheostomy and/or gastrostomy for patients having undergone DC could redirect their end-of-life goals and change the course of the patient care pathway if more knowledge about the psychosocial and physical realities of the procedure is provided. Given the life-preserving capacity of tracheostomy and gastrostomy, withdraw of medical care is the usual alternative for many patients.
Since DC is performed for patients with severe neurological injury, one could reasonably anticipate that many patients will need tracheostomy and/or gastrostomy after DC. Therefore, one could advocate for treating physicians’ discussion of these eventualities with patients and/or families as appropriate when discussing the DC procedure. With these points in mind, in this retrospective study, the authors examine the frequency of tracheostomy and/or gastrostomy after DC, as well as any relation between tracheostomy and/or gastrostomy and withdrawal of life-preserving care. The objective of the paper was to determine how often patients who survive after DC have a tracheostomy and/or gastrostomy after their surgery. The authors hypothesize that patients who survive after DC are more likely to receive a tracheostomy and/or gastrostomy in the postoperative period than those who die during the postoperative period. Furthermore, the authors hypothesize that patients (or families) choosing withdrawal of life-preserving care after DC do so by declining tracheostomy and/or gastrostomy.
| Materials and Methods | ▴Top |
Ethical considerations
This study met the requirements of the Institutional Review Board (IRB) of the University of Missouri for medical research and adhered to all established guidelines regarding the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the treatment of human subjects. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helinski Declaration. Waiver of consent (IRB number: 2022255) was granted due to the retrospective nature of the study and protection of the patients’ health care information.
Patients
Patients admitted to the University of Missouri Hospital who had neurosurgical consultation between January 1, 2014, and March 31, 2022, were entered into the daily neurosurgery census. Patients undergoing DC for trauma or stroke were identified from review of each daily census. Patients were excluded if they were pregnant, prisoners, or less than 18 years old. Patients were also excluded if DC was performed for infection or tumor.
Data collection
Name, date of admission, medical record number, and age were obtained from the census and cross referenced to the electronic medical record (EMR) (Powerchart; Cerner, North Kansas City, Missouri). Gender, diagnosis (trauma, ischemic stroke, hemorrhagic stroke), subdiagnosis (subdural hematoma, epidural hematoma, cerebral contusion, anterior circulation occlusion, posterior circulation occlusion, intraparenchymal hemorrhage, diffuse cerebral edema), admission status, Glasgow Coma Scale score (GCS) at admission, GCS just prior to decompression, presentation time, operating room presentation time, admission service (neurosurgery, trauma/surgical intensive care unit (ICU), internal medicine/medical ICU, neurology/stroke/neuroscience ICU), time to decompression, and tracheostomy and gastrostomy status were all obtained retrospectively through the EMR. Dominant hemisphere surgery was determined by the side of the craniectomy and patient handedness; for right-handed patients, the left hemisphere was considered dominant and for a left-handed person, the right hemisphere was considered dominant. Admission code status (full code, do not resuscitate (DNR) prior to decompression and DNR after decompression) was noted. Potential confounding factors considered included Injury Severity Score and Charlson score for comorbidities where appropriate.
Outcome measures
Outcomes measures included mortality; Glasgow Outcome Scale (GOS) at 3 months, 6 months, 1 year, and last follow-up; GCS prior to withdrawal of care; modified Rankin Scale (mRS) at 3 months, 6 months, 1 year and last follow-up; and cause of death (withdrawal of care while stable, withdrawal of care due to elevated ICP, withdrawal of care due to medical instability, and brain death). Admission variables for GCS were separated into groups of 3, 4 - 5, 6 - 7, 8 - 12, 13 - 15. Discharge disposition (home, inpatient rehabilitation (IPR), skilled nursing facility (SNF), long-term acute care hospital (LTAC), and deceased) was also assessed. Palliative care status was listed as those receiving comfort care measures (palliative care and/or general inpatient hospice (GIP)) and those not receiving comfort care measures. Patients who died were censured for this analysis.
Patient gender, age, race, insurance status, marital status, language, and religion were all monitored for possible biases or lack of representation.
Data analysis
Patients were placed into two groups: patients who underwent a DC and received a subsequent tracheostomy and/or gastrostomy during initial hospital admission, and patients who only underwent a DC without subsequent tracheostomy or gastrostomy. Chi-square test was used to evaluate the relationship of a tracheostomy and/or gastrostomy to patient survival after surgery, to compare categorical outcomes in variables between groups receiving a tracheostomy and/or gastrostomy to those who did not, and to test whether patients with a tracheostomy and/or gastrostomy were more likely to utilize hospice or end-of-life care. Data were also analyzed for patients who received a tracheostomy with or without gastrostomy and for those who received a gastrostomy with or without tracheostomy. Fisher’s exact test was used in place of Chi-square if any count was 5 or less for a particular outcome variable. Two-tailed t-test was used to evaluate significance of continuous variables. Multiple regression models were used to control for age, sex, race, marital status, English-speaking status, diagnosis, subdiagnosis, admission code status, dominant hemisphere surgery, decompression type, admission setting, insurance status, and presence of family member listed in the chart to assess potential confounders to determine odds ratio (OR) at the 95% confidence interval (CI) for outcomes. For all tests, significance was determined by P < 0.05.
The outcomes for both GOS and mRS were examined in three different ways. First, the GOS and mRS were examined at a 3-month, 6-month and 1-year follow-up visits with the patient. Next, the gravity of the patient’s condition was compared to their outcome by examining admission GCS and pre-operation GCS compared to death status, GOS, mRS outcomes, and cause of death by OR. Outcome data for death status, GOS, mRS, cause of death, and utilization of comfort care and hospice care were compared between patients receiving a tracheostomy and/or gastrostomy and those who did not. All statistical data analysis was conducted using SAS Enterprise Guide version 8.3® and Microsoft excel®.
| Results | ▴Top |
Demographics
Seventy patients undergoing DC were listed in the daily neurosurgical censuses between January 2014 and March 2022. Of these, 66 met the inclusion criteria. Thirty-four of these patients received a tracheostomy or gastrostomy, of which 26 patients had a tracheostomy and 29 had a gastrostomy; 32 had neither tracheostomy nor gastrostomy (Table 1). The median age of all patients was 50 years old (interquartile range (IQR): 37 - 61 (25th - 75th percentiles)). The median age of patients receiving a tracheostomy or gastrostomy was 48 years old (IQR: 33.5 - 57.5), which was not significantly different from that of for those who did not receive a tracheostomy or gastrostomy (54.5 years old, IQR: 37 - 63.5, P = 0.14). Forty-one of the patients were male and 25 were female, with no difference in gender between tracheostomy and/or gastrostomy and no tracheostomy or gastrostomy (P = 0.95). Thirty-two tracheotomy and/or gastrostomy patients had a family member listed in the chart, compared to 28 patients without tracheostomy or gastrostomy (P = 0.4202).
 Click to view | Table 1. Patient Characteristics |
DC
Nineteen patients (28.8%) underwent DC because of trauma and 47 patients (71.2%) because of stroke (ischemic stroke: 25 patients, hemorrhagic stroke: 22 patients). Sixty-two patients underwent unilateral DC, two patients underwent a bifrontal decompression, and two patients underwent a posterior fossa (suboccipital) decompression. Admission service included 25 patients (37.9%) to trauma/surgical intensive care unit (SICU), 25 patients (37.9%) to neurology/stroke/neuroscience ICU, 15 patients (22.7%) to neurosurgery, and one patient (1.5%) to internal medicine. Tracheostomy and/or gastrostomy did not differ between admission services, with 64% of trauma/surgical ICU patients, 60% of neurosurgery patients, 32% of neurology/stroke/neuroscience ICU patients, and the one internal medicine patient receiving a tracheostomy and/or gastrostomy (P = 0.08).
DC was performed on the dominant hemisphere in 22 patients (33.3%). Of these, 13 had a tracheostomy and/or gastrostomy and nine did not have tracheostomy or gastrostomy (P = 0.2102). Surgery on the dominant hemisphere did not affect mortality (P = 0.7992).
Median time from admission to DC was 19.1 h (IQR: 4.67 - 50.97) for all patients (Table 2). Median time from admission to DC was not significantly less for patients who subsequently received a tracheostomy and/or gastrostomy (17.25 h, IQR: 3 - 50.97), compared to those who did not receive a tracheostomy or gastrostomy (23.75 h, IQR: 9.64 - 48.73) (P = 0.23). The median time from surgery to receive a gastrostomy was 10 days (IQR: 7 - 16), and median time to receive a tracheostomy was 7 days (IQR: 3 - 11). The median number of days between receiving DC surgery and opting for comfort care measures was 5.5 (IQR: 2.5 - 11). Of note, the median time to receiving comfort care after DC was skewed by two patients at 430 and 74 days, and the other 11 patients opted for comfort care in median 4.5 days (IQR: 2 - 10).
 Click to view | Table 2. Timing of Events |
Forty-two patients had admission GCS between 3 and 7 (low GCS), and 24 patients had admission GCS greater than 8 (high GCS). Twenty-five low GCS patients (59.5%) received a subsequent tracheostomy/gastrostomy compared to nine high GCS patients (37.5%); these results did not differ between those with tracheostomy and/or gastrostomy and those without tracheostomy or gastrostomy (P = 0.085).
Outcomes
Thirteen patients (19.7%) received comfort care measures while under care at the University Hospital, and nine patients (13.6%) were placed on GIP services. Discharge disposition included 27 patients to IPR (40.9%), eight patients to LTAC (12.1%), six patients to home (9.1%), five patients to SNF (7.6%). Nineteen patients died at the hospital (28.8%). The main cause of death in the 19 patients was 11 patients with persistent elevated ICP leading to withdraw of care (57.9%), three patients with medical instability (15.8%), three patients with brain death (15.8%), and two patients with withdraw of care despite stable condition (10.5%) (Table 3). Only three of the patients with persistent elevated ICP (27.7%) received a tracheostomy and/or gastrostomy, while three patients who subsequently progressed to brain death, one patient who had withdrawal of care despite being stable, and no patients who were medically unstable with withdrawal of care received a tracheostomy and/or gastrostomy. Of patients who survived, 57.4% received tracheostomy and/or gastrostomy.
 Click to view | Table 3. Cause of Death |
Median GOS for those receiving a tracheostomy and/or gastrostomy was 3 (IQR: 2 - 3), 3 (IQR: 1 - 3), and 3 (IQR: 1 - 4) at 3-month, 6-month and 1-year follow-up, respectively, which were not significantly different compared to median GOS for those not receiving a tracheostomy or gastrostomy of 3 (IQR: 1 - 3), 3 (IQR: 1 - 3), and 3 (IQR: 1 - 3) at 3-month, 6-month and 1-year follow-up, respectively (P = 0.69, 0.50, and 0.13) (Fig. 1). Median mRS for those receiving a tracheostomy and/or gastrostomy was 5 (IQR: 4 - 5), 4 (IQR: 4 - 6), and 4 (IQR: 3 - 6) at 3-month, 6-month and 1-year follow-up, respectively, and were not significantly different compared to mRS for those who did not receive a tracheostomy or gastrostomy of 5 (IQR: 4 - 6), 4 (IQR: 4 - 6), and 4 (IQR: 4 - 6) at 3-month, 6-month and 1-year follow-up, respectively (P = 0.95, 0.52, and 0.10). Surgery on the dominant hemisphere did not significantly affect GOS or mRS at any time point (data not shown). Consideration of tracheostomy alone or gastrostomy alone also showed no significant relationships (data not shown).
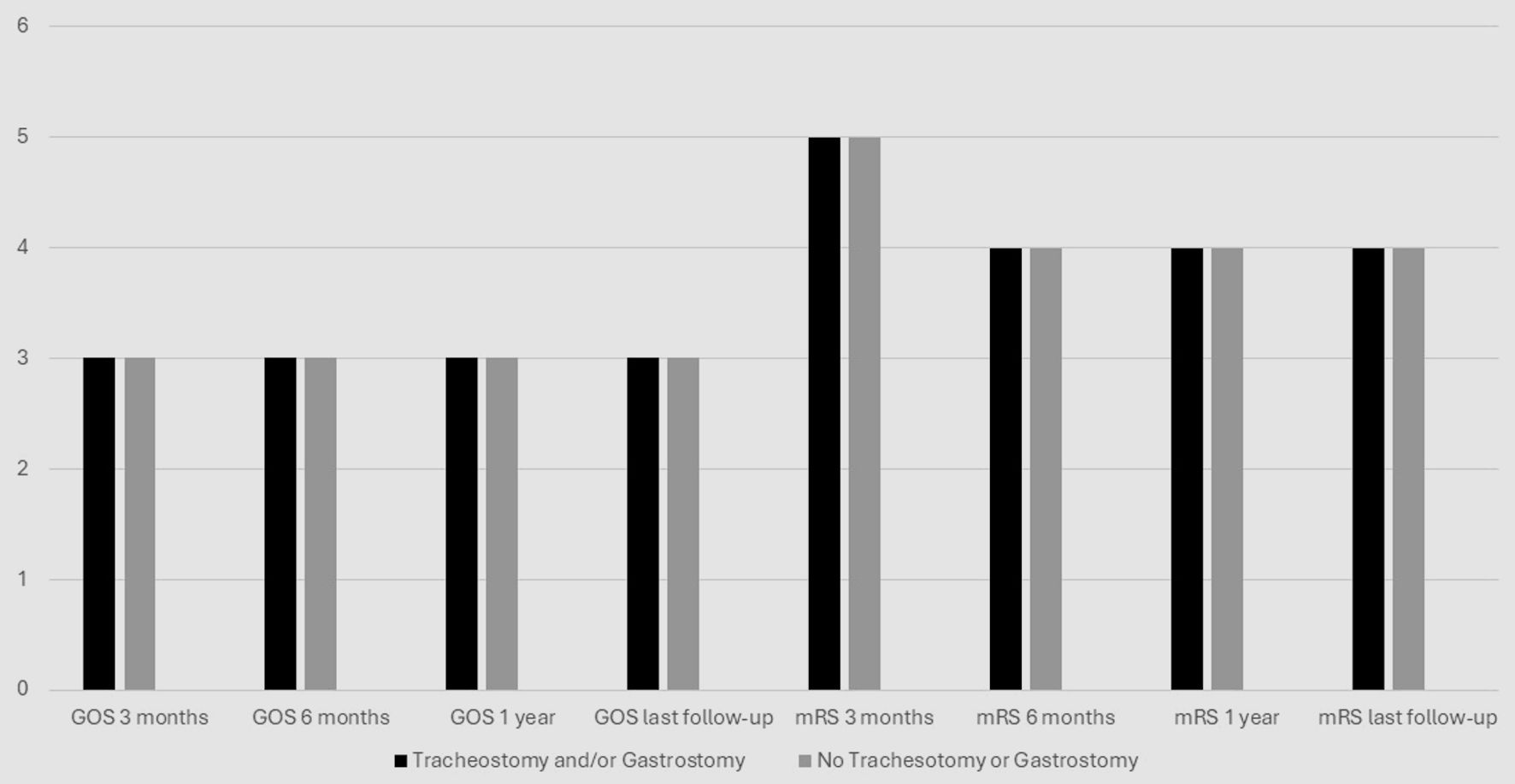 Click for large image | Figure 1. GOS/mRS outcomes over time. GOS: Glasgow Outcome Scale; mRS: modified Rankin Scale. |
End of end-of-life care
Of the 19 patients who died, six (31.6%) received a tracheostomy and/or gastrostomy while 13 (68.4%) did not (P = 0.0394) (Fig. 2). The OR from the multiple regression model comparing the mortality of patients who received a tracheostomy and/or gastrostomy and those who did not was 29.8 (95% CI: 2.25 - 395.82). Patients who did not receive a tracheostomy or gastrostomy were almost three times more likely to die in the in-patient setting than patients that did receive a tracheostomy and/or gastrostomy (P = 0.01). OR could not be calculated for the tracheostomy group and the gastrostomy group separately because of too many overlapping patients. Three of 13 comfort care patients had received a tracheostomy and/or gastrostomy (23.1%), while 10 (76.9%) did not (P = 0.0183). Of the nine patients receiving GIP care, only one received a tracheostomy and/or gastrostomy, while eight (88.9%) did not (P = 0.0091).
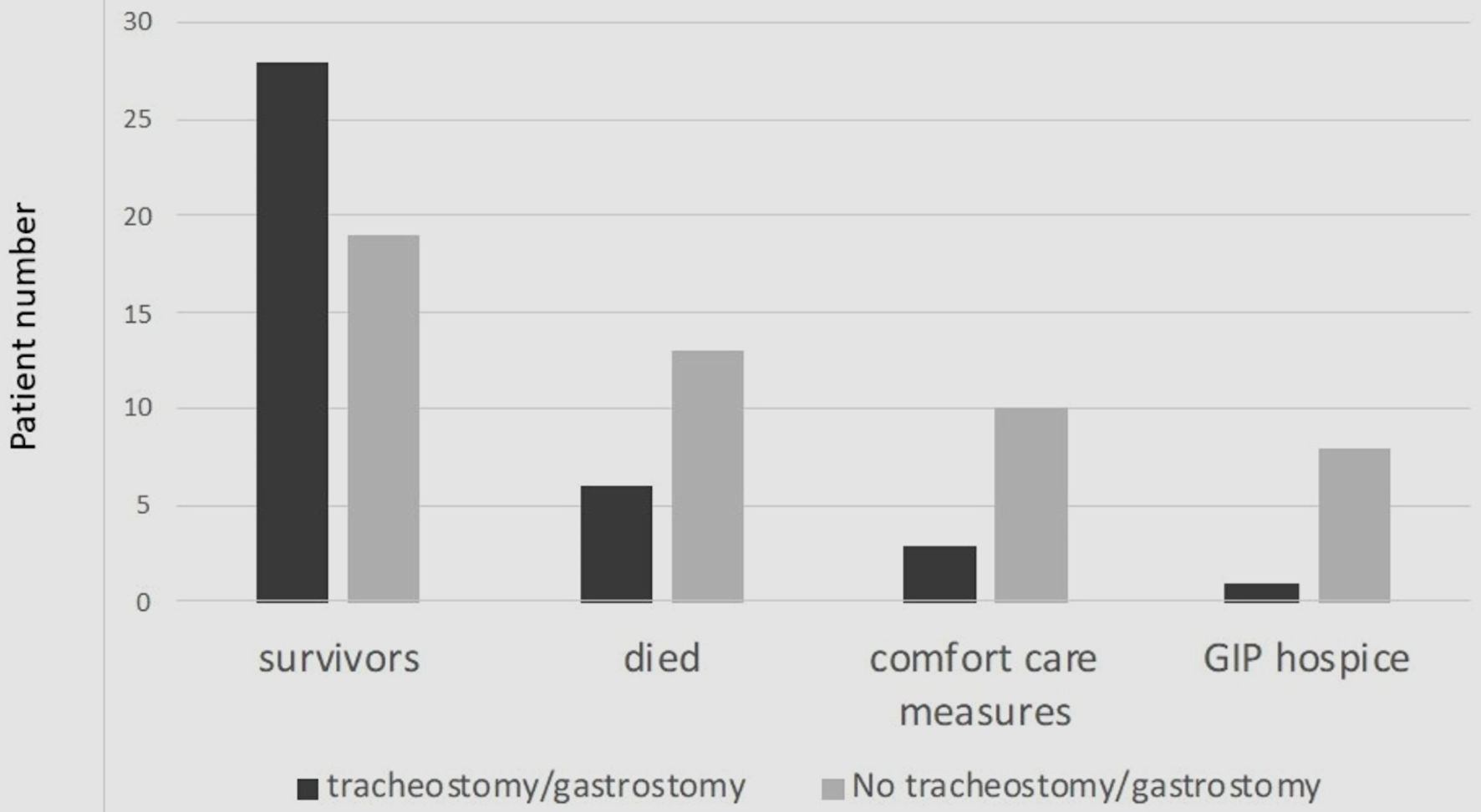 Click for large image | Figure 2. Survival outcomes. GIP: general inpatient hospice. |
| Discussion | ▴Top |
For patients who require DC for control of refractory elevated ICP or to salvage a patient with a brain herniation syndrome, subsequent tracheostomy and/or feeding gastrostomy is often needed. For instance, Guillotte et al [11] have shown that a GCS motor score of 5 on day 5 after TBI is an excellent marker for likely need for subsequent tracheostomy. This study shows that patients who survived after DC were more likely to have received tracheostomy and/or gastrostomy than those who did not survive. Furthermore, those who receive comfort measures or are placed on GIP services are more likely not to receive tracheostomy and/or gastrostomy. These data do not mean that tracheostomy and gastrostomy improve survival following DC. A more likely interpretation is that patients who have DC who have weathered their neurological insult are more likely to proceed to tracheostomy and/or gastrostomy for appropriate supportive care.
These data also show that most non-survivors died from progression of intracranial processes or severe medical complications. Only 10.5% of patients died as a result of withdrawal of care while neurologically or medically stable. This study could not discern if the decision to withdraw care was related to the need for tracheostomy or gastrostomy, but having such divergent choices does not appear to be a major factor. Therefore, while hypothesis that patients without a tracheostomy and/or gastrostomy would be more likely to have withdrawal of life-preserving care is essentially correct, the reason does not appear to be that the choice is an alternative to tracheostomy and/or gastrostomy. Rather, the choice to proceed to withdrawal of care and/or hospice appears to be made because of progression of intracranial disease processes or development of severe medical complications in most cases. Unfortunately, discussions about advanced care planning and goals of care were not well documented in the medical record to develop the evidence necessary to be certain of reasons for withdrawal of care, particularly in stable patients. Furthermore, because sample size is small, particularly for those who died, conclusions regarding cause of death cannot be made reliably.
These results suggest that tracheostomy and feeding gastrostomy are generally, but not always, a part of the subsequent care pathway for patients who require DC. As such, patients and families should be educated about the likely need for subsequent tracheostomy and/or gastrostomy as part of the consenting process for DC. This discussion is not dissimilar to approaches in amyotrophic lateral sclerosis (ALS) [12, 13] and stroke patients, even in the absence of DC [14]. Obviously, patients who improve quickly to the point of being able to protect their airway can be extubated and likely will not require tracheostomy and/or gastrostomy. Others, like the 57.4% of survivors who had tracheostomy and/or gastrostomy, should be prepared to expect those possibilities.
The data in this study do not indicate a significant difference in functional outcomes between groups with a tracheostomy and/or gastrostomy and those without. Other studies have demonstrated a lack of functional improvement with tracheostomy during mechanical ventilation in the ICU [15, 16]. By contrast, critically ill patients who receive a tracheostomy may have more favorable functional outcomes compared to those who do not [17], as tracheostomy can aid in reduction of sedation, facilitated ventilator weaning, earlier mobilization with reduced ICU-related paresis, more autonomy, and earlier participation with physical and occupational therapies. Earlier oral alimentation may improve nutritional status [18, 19]. Unfortunately, some patients after tracheostomy may be less participatory, particularly those who are depressed [8]. Therefore, functional outcome benefits of tracheostomy and/or gastrostomy remain in question and require further investigation.
Tracheostomy and gastrostomy can be associated with adverse consequences. In some studies, patient outcome is worse with tracheostomy [20, 21]. The use of tracheostomy in an ICU setting can result in poor physical function for up to 1 year after placement [22]. Likewise, mental health changes, such as depression, can affect patients’ quality of life after tracheostomy and gastrostomy placement [17]. Additionally, tracheostomy has been shown to prolong patients’ hospital stay durations [17] and prolong disability [23], although other studies refute this finding [24, 25], particularly after DC [26]. Despite these negatives, our data suggest that tracheostomy and gastrostomy have value to patients. Whether the same results could have been achieved without tracheostomy and/or gastrostomy is unknown, and we did not assess psychosocial consequences of tracheostomy and/or gastrostomy.
The observation that three patients who proceeded to brain death had had a tracheostomy suggests that potential for occasional injudicious use of the resource. One should keep in mind the course of the disease in an individual patient before subjecting that patient to invasive procedures. Tracheostomy is unlikely to alter the outcome of a patient with elevated ICP, so deferring the procedure until the patient is neurologically stable makes sense. Percutaneous tracheostomy can increase ICP and cerebral perfusion pressure; patients with a baseline ICP > 15 mmHg are at risk to develop harmful ICP crises during tracheostomy [27]. Therefore, tracheostomy should be deferred in patients with elevated ICP or who are at risk of developing elevated ICP [28].
More transparent and planned end-of-life care in trauma and stroke settings is needed [14]. Understanding the risks and benefits of such high-intensity treatment is important in providing patient-centered care. Because most patients who survive after DC often require tracheostomy and feeding gastrostomy as part of their supportive care, patients and/or families or other surrogates should be advised to expect the possibility of these procedures during the consenting process for the DC.
This single institution study has several limitations. A sample size of 66 could lead to sample size bias. Likewise, regression analysis of variables under specific subsets such as setting and tracheostomy and/or gastrostomy status yield even smaller sample sizes; for example, three patients who progressed to brain death received a tracheostomy and/or gastrostomy. Some analyses, such as that for preoperative GCS, show insignificant relationships, but these could be examples of type II errors resulting from the small sample size. Outlier bias may also be present, as the standard deviation for days to comfort care from operating room date was quite large, which may have been influenced by outlier patients. This study only reviewed the data from a single institution, limiting the generalizability of study findings. The potential impact on decision-making of the enthusiasm of the surgeon and/or intensivist for patient recovery could create selection bias; this factor was unable to be analyzed as a confounder. Selection bias is also possible; for example, postoperative GCS was not assessed. Additionally, the indications for DC and tracheostomy and/or gastrostomy were not specified as a priori in this study, and these indications may vary from institution to institution, providing differences in outcomes. Unfortunately, as this study is retrospective, factors such as whether or not the family had a prior conversation regarding the quality of life, who was the decision-maker for the DC (spouse, children, strange first kin), and which service(s) (neurosurgery, critical care, trauma, neurology) discussed DC with the family are not addressed. Risk factors for tracheostomy and gastrostomy are also not addressed. Many of these limitations could and should be addressed in a prospective study.
Conclusions
Patients who survive after DC are more likely to receive tracheostomy and/or feeding gastrostomy than those who did not survive. Patients who seek end-of-life care, including withdrawal of care and GIP, are more likely not to receive tracheostomy and or gastrostomy. The frequency of subsequent tracheostomy and/or gastrostomy suggest the importance of discussing their likelihood with patients and/or surrogates during the consenting process for DC.
Acknowledgments
The work was presented in part as a poster at the Research Day Symposium at the University of Missouri School of Medicine on November 17, 2022. The authors also wish to thank Mary Beth Litofsky, RN, MSN, for her editorial assistance.
Financial Disclosure
This work was supported by research funds from the Department of Neurosurgery of the University of Missouri School of Medicine and a Medical Student Summer Research Fellowship from the University of Missouri School of Medicine for Aidan Jacobsen, MS.
Conflict of Interest
The authors report no conflict of interest.
Informed Consent
Wavier of consent was obtained from the Institutional Review Board of the University of Missouri for medical research (IRB number: 2022255).
Author Contributions
Aidan J. Jacobsen, MS: data curation, formal analysis, investigation, methodology, and writing - original draft. Chase Schlesselman, MPH: formal analysis and methodology. Norman Scott Litofsky, MD: conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, supervision, and writing - review and editing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ALS: amyotrophic lateral sclerosis; DC: decompressive craniectomy; DNR: do not resuscitate; EMR: electronic medical record; GIP: general inpatient hospice; GCS: Glasgow Coma Scale; GOS: Glasgow Outcome Scale; ICP: intracranial pressure; ICU: intensive care unit; IPR: inpatient rehabilitation; IRB: Institutional Review Board; IQR: interquartile range; LOS: length of stay; LTAC: long-term acute care hospital; mRS: modified Rankin Scale; OR: odds ratio; SNF: skilled nursing facility; TBI: traumatic brain injury
| References | ▴Top |
- Honeybul S, Ho KM, Gillett GR. Reconsidering the role of decompressive craniectomy for neurological emergencies. J Crit Care. 2017;39:185-189.
doi pubmed - Pelosi P, Ferguson ND, Frutos-Vivar F, Anzueto A, Putensen C, Raymondos K, Apezteguia C, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39(6):1482-1492.
doi pubmed - Lilley EJ, Scott JW, Weissman JS, Krasnova A, Salim A, Haider AH, Cooper Z. End-of-life care in older patients after serious or severe traumatic brain injury in low-mortality hospitals compared with all other hospitals. JAMA Surg. 2018;153(1):44-50.
doi pubmed pmc - Chatterjee A, Chen M, Gialdini G, Reznik ME, Murthy S, Kamel H, Merkler AE. Trends in tracheostomy after stroke: analysis of the 1994 to 2013 national inpatient sample. Neurohospitalist. 2018;8(4):171-176.
doi pubmed pmc - Wabl R, Williamson CA, Pandey AS, Rajajee V. Long-term and delayed functional recovery in patients with severe cerebrovascular and traumatic brain injury requiring tracheostomy. J Neurosurg. 2018;131(1):114-121.
doi pubmed - Nakarada-Kordic I, Patterson N, Wrapson J, Reay SD. A systematic review of patient and caregiver experiences with a tracheostomy. Patient. 2018;11(2):175-191.
doi pubmed - Gilony D, Gilboa D, Blumstein T, Murad H, Talmi YP, Kronenberg J, Wolf M. Effects of tracheostomy on well-being and body-image perceptions. Otolaryngol Head Neck Surg. 2005;133(3):366-371.
doi pubmed - Carroll SM. Silent, slow lifeworld: the communication experience of nonvocal ventilated patients. Qual Health Res. 2007;17(9):1165-1177.
doi pubmed - Saricam G, Akdogan D, Kahveci K. Palliative care after stroke. Acta Neurol Belg. 2019;119(1):69-75.
doi pubmed - Ashana DC, Umscheid CA, Stephens-Shields AJ, Kohn R, Madden V, Harhay MO, Chen Y, et al. Determining the association between end-of-life care resources and patient outcomes in Pennsylvania ICUs. Crit Care Med. 2019;47(11):1591-1598.
doi pubmed pmc - Guillotte AR, Fry L, Gattozzi D, Shah K. Glasgow coma scale motor score predicts need for tracheostomy after decompressive craniectomy for traumatic brain injury. Korean J Neurotrauma. 2023;19(4):454-465.
doi pubmed pmc - Burkhardt C, Neuwirth C, Sommacal A, Andersen PM, Weber M. Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLoS One. 2017;12(5):e0177555.
doi pubmed pmc - Cui F, Sun L, Xiong J, Li J, Zhao Y, Huang X. Therapeutic effects of percutaneous endoscopic gastrostomy on survival in patients with amyotrophic lateral sclerosis: A meta-analysis. PLoS One. 2018;13(2):e0192243.
doi pubmed pmc - Gao L, Zhao CW, Hwang DY. End-of-life care decision-making in stroke. Front Neurol. 2021;12:702833.
doi pubmed pmc - Scales DC, Thiruchelvam D, Kiss A, Redelmeier DA. The effect of tracheostomy timing during critical illness on long-term survival. Crit Care Med. 2008;36(9):2547-2557.
doi pubmed - Cinotti R, Voicu S, Jaber S, Chousterman B, Paugam-Burtz C, Oueslati H, Damoisel C, et al. Tracheostomy and long-term mortality in ICU patients undergoing prolonged mechanical ventilation. PLoS One. 2019;14(10):e0220399.
doi pubmed pmc - Kollef MH, Ahrens TS, Shannon W. Clinical predictors and outcomes for patients requiring tracheostomy in the intensive care unit. Crit Care Med. 1999;27(9):1714-1720.
doi pubmed - Nieszkowska A, Combes A, Luyt CE, Ksibi H, Trouillet JL, Gibert C, Chastre J. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005;33(11):2527-2533.
doi pubmed - Heffner JE, Hess D. Tracheostomy management in the chronically ventilated patient. Clin Chest Med. 2001;22(1):55-69.
doi pubmed - Kaushal A, Bindra A, Kumar A, Goyal K, Kumar N, Rath GP, Gupta D. Long term outcome in survivors of decompressive craniectomy following severe traumatic brain injury. Asian J Neurosurg. 2019;14(1):52-57.
doi pubmed pmc - Laghari AA, Bari ME, Waqas M, Ahmed SI, Nathani KR, Moazzam W. Outcome of decompressive craniectomy in traumatic closed head injury. Asian J Neurosurg. 2018;13(4):1053-1056.
doi pubmed pmc - Scheinhorn DJ, Hassenpflug MS, Votto JJ, Chao DC, Epstein SK, Doig GS, Knight EB, et al. Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131(1):85-93.
doi pubmed - Luta X, Maessen M, Egger M, Stuck AE, Goodman D, Clough-Gorr KM. Measuring intensity of end of life care: a systematic review. PLoS One. 2015;10(4):e0123764.
doi pubmed pmc - Hemmati H, Forozeshfard M, Hosseinzadeh B, Hemmati S, Mirmohammadkhani M, Bandari R. Tracheostomy in patients who need mechanical ventilation: early or late? Surgical or percutaneous? A prospective study in Iran. Indian J Surg. 2017;79(5):406-411.
doi pubmed pmc - Dochi H, Nojima M, Matsumura M, Cammack I, Furuta Y. Effect of early tracheostomy in mechanically ventilated patients. Laryngoscope Investig Otolaryngol. 2019;4(3):292-299.
doi pubmed pmc - Qureshi MSS, Shad ZS, Shoaib F, Munawar K, Saeed ML, Hussain SW, Qadeer A, et al. Early versus late tracheostomy after decompressive craniectomy. Cureus. 2018;10(12):e3699.
doi pubmed pmc - Kleffmann J, Pahl R, Ferbert A, Roth C. Factors influencing intracranial pressure (ICP) during percutaneous tracheostomy. Clin Neurol Neurosurg. 2020;195:105869.
doi pubmed - Kirkman MA, Smith M. Intracranial pressure monitoring, cerebral perfusion pressure estimation, and ICP/CPP-guided therapy: a standard of care or optional extra after brain injury? Br J Anaesth. 2014;112(1):35-46.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
