| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 3, Number 3-4, August 2013, pages 108-113
Endurance Exercise Training Attenuates the up Regulation of iNOS in the Skeletal Muscles of Chronic/Progressive Mouse Model of Parkinson’s Disease
Nour Erekata, Ahed Al Khatibb, Muhammed Al-Jarrahc, d
aDepartment of Anatomy, Faculty of Medicine, Jordan University of Science and Technology, JUST, Jordan
bDepartment of Pathology, Faculty of Medicine, JUST, Jordan
cDepartment of Rehabilitation Sciences, Faculty of Applied Medical Sciences, JUST, Jordan
dCorresponding author: Muhammed Al-Jarrah, Jordan University of Science and Technology, Faculty of Applied Medical Sciences, Department of Rehabilitation Sciences, 22110 P. O box 3030. Irbid, Jordan
Manuscript accepted for publication July 19, 2013
Short title: iNOS Expression in PD Skeletal Muscles
doi: https://doi.org/10.4021/jnr217w
| Abstract | ▴Top |
Background: Parkinson’s disease (PD) is one of the most common neurodegenerative diseases in the elderly. PD complications include muscle weakness and fatigue, these complications lead in part to decrease the endurance of PD patients. Strength of skeletal muscles has a major role in ADL performance, which is one of the challenges that PD patients have. The main goals of this study are to study the expression of inducible nitric oxide synthase (iNOS) in the skeletal muscles of PD and to examine the effect of treadmill exercise training on iNOS expression in these skeletal muscles.
Methods: Twenty normal albino mice and 20 albino mice with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) -induced PD were divided into four groups: sedentary control (SC), exercised control (EC), sedentary PD (SPD), and EPD (EPD). Parkinsonism was induced by injections of10 doses of MPTP (25 mg/kg) and probenecid (250 mg/kg) over 5 weeks. After the completion of treadmill exercise training, samples from the gastroc and soleus muscles were evaluated by immunohistochemistry to examine the expression of iNOS in the four groups of animals.
Results: Expression of iNOS in gastrocnemius muscle showed significant increase in expression of iNOS is SPD group compared to SC, # P value < 0.05. In soleus muscle, there was an increase in expression of iNOS is SPD group compared to SC, but the change was not significant, P value < 0.08. Also, exercise did not significantly decrease the expression of iNOS in Parkinsonian group P value < 0.13.
Conclusion: Our present data suggest that endurance exercise training reduces PD-induced alterations in iNOS expression in skeletal muscles. These results might beimportant in considering rehabilitation protocols for PD and its related pathophysiology.
Keywords: Parkinson’s disease; iNOS; Skeletal muscles
| Introduction | ▴Top |
Parkinson disease is a common neurodegenerative disorder caused by significant depletion in dopamine, which leads to abnormal voluntary movements produced by skeletal muscles [1]. Nitric Oxide Synthase (NOS) serves as a key signaling molecule in physiological process that includes immune system defense, neuronal communications, and regulation of vascular injuries [2].iNOS protein was found post-mortem in the brains of patients with Alzheimer’s and PD [2]. In PD, ambulation is one of the major concerns, but difficulties and abnormalities in gait are mostly attributed to neuronal issues not to the contractile capacity of the muscles [3]. Recently, literature also has focused in non motor issues in PD including, muscles weakness, decreased strength, and decreased endurance. These studies suggested that exercise might be the mediator that improves the neuronal communication in PD [4].
The 1-methyl-4-1, 2, 3, 6-tetrahydropyridine (MPTP) induces Parkinsonism due to its production of reactive oxygen species, peroxynitrite, which eventually leads to the nitration of tyrosine residues and the subsequent depletion of dopamine [1-3]. Previous studies [5] strongly suggested a harmful upregulation of inducible nitric oxide synthase (iNOS) in the brain of animals and humans following MPTP administration. Moreover, iNOS has been reported to be involved in the muscle loss occurring in muscle wasting syndromes like sarcopenia and cachexia [6].
We hypothesize that iNOS plays a pathological role in the skeletal muscle abnormalities observed in PD. Therefore, using immunohistochemistry and light microscopy, our study has investigated the expression of iNOS in fast-twitch (gastrocnemius) and slow-twitch skeletal muscles (soleus) of mice with MPTP/probencid-induced chronic PD. In addition to that, our study has revealed the impact of exercise on PD-induced alteration in iNOS expression in soleus and gastrocnemius muscles.
| Materials and Methods | ▴Top |
Animals
Twenty normal Albino mice and 20 Albino mice with MPTP-induced Parkinson disease were used. The animals were housed in individual cages under identical conditions (22 ± 1 °C, free access to standard chow and water, 12 hours dark/light cycle). Animal-related protocols were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Jordan University of Science and Technology.
The 40 mice were divided in 4 equal groups, which were: sedentary control (SC, n = 10), exercised control (EC, n =10), sedentary PD (SPD, n = 10), and exercised PD (EPD, n = 10). Parkinsonism was induced by mice with 10 doses of MPTP (25 mg/kg) and probenecid (250 mg/kg) purchased from Sigma Chemical Co. (St. Louis, MO, USA) over 5 weeks, three days and half apart. Control mice received (25 mg/kg) saline injections.
Exercise protocol
Animals were introduced to modified human treadmill in which each animal run in separate chamber divided by glass so running animals can see each other. Ten chambers were placed on the belt of human treadmill, so 10 animals can exercise in each session of training. Animals were exercised for 40 minutes per day, 5 days per week for 4 weeks at a speed of 18 m/min. Although sedentary mice did not exercise, they were exposed to the same environment as the exercised mice by transferring them to the training room daily.
iNOS immunostaining of skeletal muscles
The mice were sacrificed, and their skeletal muscles (gastrocnemius/soleus) were dissected and fixed in 4% parafolmaldehyde, embedded in paraffin, and sliced in 5 micrometer thick sections. Then, the 5 µm thick sections were processed via immunohistochemistry using an antibody to iNOS (Biocare medical, San Antonio, TX). So, the five micron thick paraffin-embedded sections mounted on glass slides were deparaffinized in xylene for 2 minutes twice, and subsequently rehydrated through serially descending dilutions of alcohol (starting with 100%, and ended with 70%) followed by water (2 minutes for each step). After that, sections were processed for antigen retrieval in the reveal solution (RV 1000M, Biocare Medical, Concord, CA) under pressure in the Decloaking chamber (Biocare medical) for 2 minutes. Tissue sections were then cooled down to room temperature, and incubated with 3% hydrogen peroxidase in methanol for 5 minutes. After washing the sections in phosphate buffered saline (PBS), they were incubated with iNOS antibody (Biocare medical, San Antonio, TX), with the dilution recommended by the vendor, at room temperature for one hour. Next, the sections were washed in PBS and incubated with biotinylated secondary antibody (LSAB kit, Dako Carpinteria, CA) for 15 minutes at room temperature, then washed with PBS. Then, sections were incubated with streptavidin horse radish peroxidase (LSAB kit, Dako) for 15 minutes at room temperature and washed with PBS, 3’-Diaminobenzidine (DAB) applied for 2 m inutes or longer, until the desired intensity was developed, and then the slides were washed with tap water to stop the reaction. Negative control sections were processed without the primary antibody. All sections were then counterstained with hematoxylin and viewed under the light microscope. Ten slides of gastrocnemius muscle and ten other slides of soleus muscle from each animal group were evaluated for iNOS expression by immunohistochemistry.
Data analysis
The sections were photographed with digital camera. Photoshop software was used. The slides from each group were analyzed by counting the total pixels area occupied by positive staining. iNOS expression was analyzed, in the different skeletal muscles, and statistically compared among the 4 different groups using paired and unpaired student t-test. Differences in iNOS expression were considered statistically significant at P value < 0.05.
| Results | ▴Top |
To investigate the effect of PD as pathology on skeletal muscles, we compared the expression of iNOS expression in both SC and SPD groups. Expression of iNOS in gastrocnemius muscle showed significant increase in expression of iNOS is SPD group compared to SC, P value <0.05. Exercise did not significantly decrease the expression of iNOS in control group P value < 0.18. However, exercise significantly decreased iNOS expression in PD-induced group, P value < 0.01 (Fig. 1). Concerning soleus muscle, results showed an increase in expression of iNOS in SPD group compared to SC, but the change was not significant, P value < 0.08. Also, exercise did not significantly decrease the expression of iNOS in Parkinsonian group, P value < 0.13 (Fig. 2).
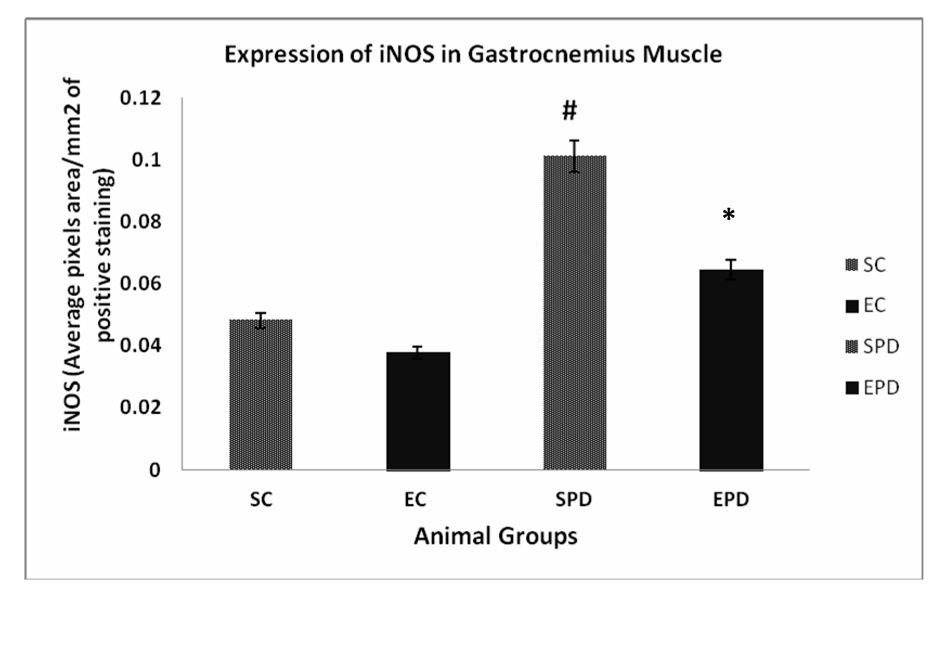 Click for large image | Figure 1. Expression of iNOS in gastrocnemius muscle showed significant increase in expression of iNOS is SPD group compared to SC, # P value < 0.05. Exercise did not significantly decrease the expression of iNOS in control group P value < 0.18. However, exercise significantly decreased iNOS expression in PD-induced group, * P value < 0.01. iNOS: Inducible nitric oxide sysnthase. SC: Sedentary control, EC: Exercised control, SPD: Sedentary Parkinson’s disease, EPD: Exercised Parkinson’s disease. |
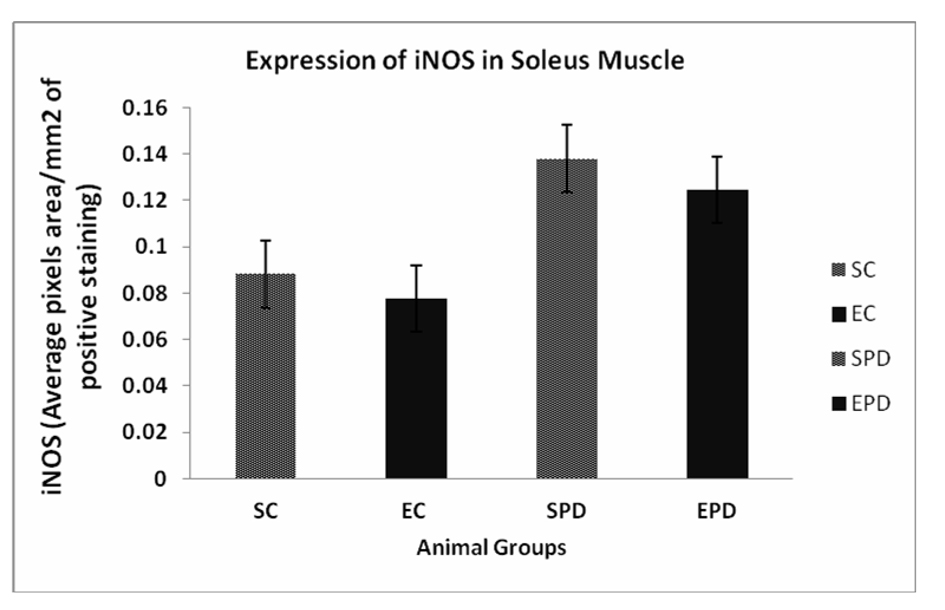 Click for large image | Figure 2. Expression of iNOS in Soleus muscle showed an increase in expression of iNOS in SPD group compared to SC, but the change was not significant #P value < 0.08. Also, exercise did not significantly decrease the expression of iNOS in Parkinsonian group P value < 0.13. iNOS: Inducible nitric oxide synthase. SC: Sedentary control, EC: Exercised control, SPD: Sedentary Parkinson’s disease, EPD: Exercised Parkinson’s disease. |
| Discussion | ▴Top |
Our study reveals two main findings. Firstly, PD increased iNOS expression in the soleus and gastrocnemius muscles. Secondly, exercise training significantly decreased iNOS expression in the gastrocnemius muscle in the Parkinsonianian mice. Thus, we postulate that there is a muscle fiber type-specific iNOS response to PD with endurance exercise training.
iNOS has been reported to be expressed at low levels in normal rodents skeletal muscles [7-12]. NOS mediates the production of (NO), which is a vasodilator important in controlling blood pressure in skeletal muscles [13, 14]. A previous report [15] has suggested no significant increase in iNOS expression in the soleus muscle following endurance exercise training. No iNOS induction in gastrocnemius muscle has been reported [16] in response to endurance exercise training. Consistent with those previous reports [15, 16], our results demonstrate that endurance exercise training does not induce any significant increase in the iNOS expression in both soleus and gastrocnemius muscles in the control mice.
To our knowledge, this is one of the first studies that examine PD-induced alterations in iNOS expression in the different types of skeletal muscles. Previous studies [17-20] have demonstrated iNOS overexpression in skeletal muscles of chronic heart failure patients and animals. Such iNOS overexpression has been shown to be induced by inflammatory cytokines and the priming effect of gamma interferone (γ-IFN) in chronic heart failure [21], iNOS overexpression has also been demonstrated in skeletal muscles of autoimmune inflammatory myopathies [22]. NOS activity inhibits mitochondrial respiration in skeletal muscles [23, 24]. iNOS overxpression results in the production of excessive NO [25]. Excessive NO causes oxidative stress [26, 27], which may be contributing to the skeletal muscle abnormalities seen in PD animals and humans.
Aging-induced iNOS upregulation has been shown [28] in the white, fast-twitch gastrocnemius skeletal muscle. These findings were consistent with our study, which revealed that PD-induced iNOS upegulation was detected more in gastrocnemiu muscle (Fig. 1). Skeletal muscle abnormalities in PD have been reported [29] to include myopathies with mitochondrial abnormalities. Mitochondrial reactive oxygen species have been demonstrated [30] to promote the production of proinflammatory cytokines, which in turn dramatically induce iNOS expression. Thus, we can conclude that iNOS upregulation detected in the parkinsonian gastrocnemius muscle maybe attributed to the difference in mitochondrial abnormalities, or the inflammatory respond in these different muscle fibers.
It has been demonstrated [21, 31-37] that iNOS overexpression is induced by various signaling molecules such as proinflammatory cytokines. Previous studies [38-41] suggested that iNOS participated in many pathological conditions. For example, iNOS has been shown to be deleterious to ischemia/reperfusion injury in skeletal muscle [42]. Selective inhibition of iNOS has been shown [42] to protect tissues against iNOS ischemia/reperfusion injury in skeletal muscle. The observed overexpression of iNOS PD skeletal muscles is consistent with the previous report [29] that skeletal muscle abnormalities in PD include inflammatory myopathies and myopathies associated with mitochondrial abnormalities. Upregulation of iNOS in PD skeletal muscles is also in agreement with the previous studies [43-45] reporting iNOS overexpression in other parkinsonian tissues including the brain, where iNOS upregulation has been suggested [46] as the cause of dopaminergic neuronal death leading to PD. Indeed, inhibition of nitric oxide synthase has been shown [45, 47, 48] to protect against MPTP-induced neurotoxicity in animals. Therefore, reducing iNOS expression either pharmacologically [49, 50], genetically [48, 51], or by exercise as our finding indicated can protect various tissues in the different pathological conditions including PD. Clinical inflammatory myopathies and myopathies associated with mitochondrial abnormalities increase in PD [29].Thus, we assume that mitochondrial abnormality-associated inflammatory cytokines are the reason behind statistically significant and statistically insignificant iNOS upregulation in parkinsonian soleus and gastrocnemius muscles.
In Rodents, fast-twitch red muscle has a higher content of mitochondria than slow-twitch red muscle. Previous reports [52-54] have suggested that exercise training promotes mitochondria biogenesis by increasing mitochondrial number, content, volume and function. Hence, we can postulate that exercise training may ameliorate the oxidative environment caused by the mitochondrial abnormalities in the PD skeletal muscles. Therefore, it can be concluded that the detected reduction in iNOS overexpression in the Parkinsonian slow-twitch (soleus) skeletal muscle following endurance exercise training is due to the expected downregulation in the signaling inflammatory cytokines resulting from mitochondrial biogenesis. The other factor that might be responsible for this difference could relate to the differences in the type of motor neuron innervating fast-twitch and slow-twitch muscle fibers that accompanied with differences in firing pattern, contractile response, and specific mechanical stress.
In summary, our study is one of the first studies to report fiber type-specific alterations in iNOS expression in PD skeletal muscles. In addition, this is the first study to examine the impact of endurance exercise training on iNOS expression in PD slow- and fast-twitch skeletal muscles.
Acknowledgments
This study was financially supported by The Deanship of Research at Jordan University of Science and Technology. Irbid, Jordan.
Declaration
We confirm that there is no known conflict of interest associated with this manuscript and there has been no financial support for this work that influenced the outcome.
| References | ▴Top |
- Tinazzi M, Recchia S, Simonetto S, Tamburin S, Defazio G, Fiaschi A, Moretto G, et al. Muscular pain in Parkinson's disease and nociceptive processing assessed with CO2 laser-evoked potentials. Mov Disord. 2010;25(2):213-220.
doi pubmed - Vodovotz Y, Lucia MS, Flanders KC, Chesler L, Xie QW, Smith TW, Weidner J, et al. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer's disease. J Exp Med. 1996;184(4):1425-1433.
doi pubmed - Vajdovich P. Free radicals and antioxidants in inflammatory processes and ischemia-reperfusion injury. Vet Clin North Am Small Anim Pract. 2008;38(1):31-123, v.
doi pubmed - Moreno Catala M, Woitalla D, Arampatzis A. Central Factors Explain Muscle Weakness in Young Fallers With Parkinson's Disease. Neurorehabil Neural Repair. 2013.
doi pubmed - Lima LO, Scianni A, Rodrigues-de-Paula F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson's disease: a systematic review. J Physiother. 2013;59(1):7-13.
doi - Stevens-Lapsley J, Kluger BM, Schenkman M. Quadriceps muscle weakness, activation deficits, and fatigue with Parkinson disease. Neurorehabil Neural Repair. 2012;26(5):533-541.
doi pubmed - Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162(3):401-409.
doi pubmed - Boczkowski J, Lanone S, Ungureanu-Longrois D, Danialou G, Fournier T, Aubier M. Induction of diaphragmatic nitric oxide synthase after endotoxin administration in rats: role on diaphragmatic contractile dysfunction. J Clin Invest. 1996;98(7):1550-1559.
doi pubmed - Hussain SN, Giaid A, El Dawiri Q, Sakkal D, Hattori R, Guo Y. Expression of nitric oxide synthases and GTP cyclohydrolase I in the ventilatory and limb muscles during endotoxemia. Am J Respir Cell Mol Biol. 1997;17(2):173-180.
doi pubmed - Park CS, Park R, Krishna G. Constitutive expression and structural diversity of inducible isoform of nitric oxide synthase in human tissues. Life Sci. 1996;59(3):219-225.
doi - Tews DS, Goebel HH, Schneider I, Gunkel A, Stennert E, Neiss WF. Expression of different isoforms of nitric oxide synthase in experimentally denervated and reinnervated skeletal muscle. J Neuropathol Exp Neurol. 1997;56(12):1283-1289.
doi pubmed - Thompson M, Becker L, Bryant D, Williams G, Levin D, Margraf L, Giroir BP. Expression of the inducible nitric oxide synthase gene in diaphragm and skeletal muscle. J Appl Physiol. 1996;81(6):2415-2420.
pubmed - Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86(9):3375-3378.
doi pubmed - Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2(8670):997-1000.
doi - Harris MB, Mitchell BM, Sood SG, Webb RC, Venema RC. Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol. 2008;104(5):795-802.
doi pubmed - Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol. 2003;284(3):L452-457.
pubmed - Adams V, Jiang H, Yu J, Mobius-Winkler S, Fiehn E, Linke A, Weigl C, et al. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33(4):959-965.
doi - Hambrecht R, Adams V, Gielen S, Linke A, Mobius-Winkler S, Yu J, Niebauer J, et al. Exercise intolerance in patients with chronic heart failure and increased expression of inducible nitric oxide synthase in the skeletal muscle. J Am Coll Cardiol. 1999;33(1):174-179.
doi - Adams V, Yu J, Mobius-Winkler S, Linke A, Weigl C, Hilbrich L, Schuler G, et al. Increased inducible nitric oxide synthase in skeletal muscle biopsies from patients with chronic heart failure. Biochem Mol Med. 1997;61(2):152-160.
doi pubmed - Riede UN, Forstermann U, Drexler H. Inducible nitric oxide synthase in skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 1998;32(4):964-969.
doi - Adams V, Nehrhoff B, Spate U, Linke A, Schulze PC, Baur A, Gielen S, et al. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54(1):95-104.
doi - Tews DS, Goebel HH. Cell death and oxidative damage in inflammatory myopathies. Clin Immunol Immunopathol. 1998;87(3):240-247.
doi - King CE, Melinyshyn MJ, Mewburn JD, Curtis SE, Winn MJ, Cain SM, Chapler CK. Canine hindlimb blood flow and O2 uptake after inhibition of EDRF/NO synthesis. J Appl Physiol. 1994;76(3):1166-1171.
pubmed - Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun. 1995;211(2):375-381.
doi pubmed - Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150(11):5080-5085.
pubmed - Dawson VL, Dawson TM. Nitric oxide in neurodegeneration. Prog Brain Res. 1998;118:215-229.
doi - Hewett SJ, Csernansky CA, Choi DW.Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron. 1994;13(2):487-494.
doi - Song W, Kwak HB, Kim JH, Lawler JM. Exercise training modulates the nitric oxide synthase profile in skeletal muscle from old rats. J Gerontol A Biol Sci Med Sci. 2009;64(5):540-549.
doi pubmed - Gdynia HJ, Sperfeld AD, Unrath A, Ludolph AC, Sabolek M, Storch A, Kassubek J. Histopathological analysis of skeletal muscle in patients with Parkinson's disease and 'dropped head'/'bent spine' syndrome. Parkinsonism Relat Disord. 2009;15(9):633-639.
doi pubmed - Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med. 2011;208(3):519-533.
doi pubmed - Chu SC, Marks-Konczalik J, Wu HP, Banks TC, Moss J. Analysis of the cytokine-stimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters. Biochem Biophys Res Commun. 1998;248(3):871-878.
doi pubmed - Dwyer-Nield LD, Srebernak MC, Barrett BS, Ahn J, Cosper P, Meyer AM, Kisley LR, et al. Cytokines differentially regulate the synthesis of prostanoid and nitric oxide mediators in tumorigenic versus non-tumorigenic mouse lung epithelial cell lines. Carcinogenesis. 2005;26(7):1196-1206.
doi pubmed - Kwon S, George SC. Synergistic cytokine-induced nitric oxide production in human alveolar epithelial cells. Nitric Oxide. 1999;3(4):348-357.
doi pubmed - Kwon S, Newcomb RL, George SC. Mechanisms of synergistic cytokine-induced nitric oxide production in human alveolar epithelial cells. Nitric Oxide. 2001;5(6):534-546.
doi pubmed - Marks-Konczalik J, Chu SC, Moss J. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kappaB-binding sites. J Biol Chem. 1998;273(35):22201-22208.
doi pubmed - Bedard S, Marcotte B, Marette A. Cytokines modulate glucose transport in skeletal muscle by inducing the expression of inducible nitric oxide synthase. Biochem J. 1997;325(Pt 2)(487-493.
- Williams G, Brown T, Becker L, Prager M, Giroir BP. Cytokine-induced expression of nitric oxide synthase in C2C12 skeletal muscle myocytes. Am J Physiol. 1994;267(4 Pt 2):R1020-1025.
pubmed - Hsieh YH, Su IJ, Lei HY, Lai MD, Chang WW, Huang W. Differential endoplasmic reticulum stress signaling pathways mediated by iNOS. Biochem Biophys Res Commun. 2007;359(3):643-648.
doi pubmed - Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, et al. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993;90(7):3024-3027.
doi pubmed - Htain WW, Leong SK, Ling EA. In vivo expression of inducible nitric oxide synthase in supraventricular amoeboid microglial cells in neonatal BALB/c and athymic mice. Neurosci Lett. 1997;223(1):53-56.
doi - Aono K, Isobe K, Kiuchi K, Fan ZH, Ito M, Takeuchi A, Miyachi M, et al. In vitro and in vivo expression of inducible nitric oxide synthase during experimental endotoxemia: involvement of other cytokines. J Cell Biochem. 1997;65(3):349-358.
doi - Qi WN, Chen LE, Zhang L, Eu JP, Seaber AV, Urbaniak JR. Reperfusion injury in skeletal muscle is reduced in inducible nitric oxide synthase knockout mice. J Appl Physiol. 2004;97(4):1323-1328.
doi pubmed - Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, Hirsch EC. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72(2):355-363.
doi - Wallace MN, Geddes JG, Farquhar DA, Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to beta-amyloid plaques. Exp Neurol. 1997;144(2):266-272.
doi pubmed - Li M, Dai FR, Du XP, Yang QD, Chen Y. Neuroprotection by silencing iNOS expression in a 6-OHDA model of Parkinson's disease. J Mol Neurosci. 2012;48(1):225-233.
doi pubmed - Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5(12):1403-1409.
doi pubmed - Snyder SH. No NO prevents parkinsonism. Nat Med. 1996;2(9):965-966.
doi pubmed - Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74(5):2213-2216.
doi pubmed - Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98(25):14669-14674.
doi pubmed - Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett. 2001;315(1-2):61-64.
doi - Cuzzocrea S, Mazzon E, Calabro G, Dugo L, De Sarro A, van De LF, Caputi AP. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med. 2000;162(5):1859-1866.
doi pubmed - Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534-540.
doi pubmed - Zhang Q, Wu Y, Zhang P, Sha H, Jia J, Hu Y, Zhu J. Exercise induces mitochondrial biogenesis after brain ischemia in rats. Neuroscience. 2012;205:10-17.
- Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012;40(3):159-164.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
