| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 10, Number 5, October 2020, pages 177-182
Impact of Cervical Collar and Patient Position on the Cerebral Blood Flow
David Wamplera, f, Brian Eastridgeb, Rena Summersc, Preston Lovec, Anand Dhariad, Ali Seifie
aDepartment of Emergency Health Sciences, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
bDepartment of Surgery, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
cSouthwest Texas Regional Advisory Council, San Antonio TX, USA
dDepartment of Neurosurgery, Kansas University Medical Center, Kansas City, MO, USA
eDepartment of Neurosurgery, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
fCorresponding Author: David Wampler, UTHSCSA Emergency Health Sciences, 4201 Medical Drive, Suite 120, San Antonio, TX 78229, USA
Manuscript submitted July 8, 2020, accepted July 15, 2020, published online August 2, 2020
Short title: C-Collar and Patient Position on the CBF
doi: https://doi.org/10.14740/jnr611
| Abstract | ▴Top |
Background: Spinal protection during emergency medical service (EMS) transport after trauma has become a focus of debate. Historically, patients at risk for spine injury are transported in a rigid collar, long spineboard and headblocks. The cervical collar (c-collar) is hypothesized to provide stabilization for the cervical spine. However, little is known how the c-collar affects cervical blood flow.
Methods: Cerebral blood flow was measured in multiple conditions using a non-invasive cerebral blood flow monitor to establish cerebral blood flow index (CBFI). The CBFI data were collected at: standing, sitting, 45°, 30°, 10° or 15°, and supine, with and without c-collar. Descriptive statistics were used for CBFI in each condition, and parametric statistical methods were utilized to determine the significance of changes in CBFI.
Results: Five volunteers were recruited, and each tested in six positions with and without c-collar. Mean age was 49 (standard deviation (SD) 15) years and 60% were male. The CBFI mean of means was 71.0 with and 69.4 without the c-collar. Only one subject demonstrated a statistically significant difference in CBFI with c-collar. The CBFI mean of means for position was 72.6 for head of bed less than 30° and 68.1 for greater than 30°. All subjects demonstrated > 99% confidence for a statistically significant difference in CBFI when dichotomized using head of bed at 30°.
Conclusions: Head of the bed position has greater influence on CBFI than the c-collar . Clinical significance in healthy volunteers is unknown but this change in cerebral blood flow may have clinical significance in traumatic brain injury or neurologic conditions that compromise autoregulation.
Keywords: Cervical collars; Head of bed elevation; Autoregulation; Cerebral perfusion; Cerebral blood flow
Key Points
- Head of the bed position impacts cerebral blood flow index.
- Application of cervical collar may not impact cerebral blood index in intact subjects.
- Initial management of trauma patients should prioritize maintenance of cerebral perfusion.
| Introduction | ▴Top |
The process of protecting the spine during emergency medical service (EMS) transport after a traumatic event has become a contemporary focus of national attention in emergency medical services [1, 2]. Historically, patients that have a suspected spinal fracture have been placed in a rigid cervical collar (c-collar), positioned supine on a rigid long spinal board (LSB), with the head secured by some type of padded foam headblocks [3]. This process was executed on all potential civilian patients with suspected spine trauma regardless the mechanism of injury. Further, this transport mode was also recommended for any patient with traumatic head injury due to the potential for concomitant cervical spine injury [4, 5].
During transport, a potential spinal trauma victim is subjected to multi-vectoral forces in all three axes of movement. The LSB is intended to lessen the movement during extrication and transport, but does come with some significant limitations [6]. One of the significant limitations is that the subject must remain supine or very nearly supine during transport. During ambulance acceleration and breaking, the weight of the patient has a tendency to shift, placing mechanical stress on the cervical spine adding potential risk to exacerbate cervical spine and spinal cord injury. The rigid c-collar is intended to stabilize the cervical spine and minimize the effect of mechanical forces acting upon the axial skeleton during transport [7].
C-collars are designed to fit around the neck to prevent movement of the head and to provide support to the effect of axial loading. Properly sizing a c-collar is not always an easily accomplished task [8]. The collar must be fitted for proper height and proper tightness. Too loose a fit and the collar loses functionality; too tight a fit and there are possible hemodynamic consequences as well as a decrease in patient comfort and compliance with the device. To maximize effectiveness, the c-collar must be properly fitted in order to provide the needed support without impinging blood flow into, or out of, the head or airflow into, and out of, the chest. Currently, there are no guidelines to direct the optimum tightness of the c-collar and the best position of injured subjects during transportation. In this study we sought to determine the effect of c-collar application and subject positioning on the cerebral blood flow.
| Materials and Methods | ▴Top |
Study design
This study was conducted as a serial N-of-one study, where the cerebral blood flow index (CBFI) was monitored for each subject under each condition in the study. Each subject was only compared to himself/herself. This study was reviewed and approved by the Institutional Review Board at the University of Texas Health Science Center at San Antonio; and this study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Instrumentation
CBFI was measured with acousto-optic monitoring utilizing ultrasound (US)-tagged light (UTLight™). This is a form of optical contrast measurement using a combination of highly coherent near-infrared laser light spectroscopy (NIRS) coupled with US by the c-FLOW™ monitor (Ornim Medical, Kfar Saba, Israel) [9]. The Ornim c-FLOW™ monitor is Food and Drug Administration (FDA) cleared for continuous non-invasive real-time cerebral blood flow monitoring. This monitor was applied to assess the changes of blood flow to the brain: before and after application of several c-collars, as the patient positions change from supine (with and without LSB), and as the head of the bed is elevated. The patient interface is via a surface sensor that is adhered to the skin (Fig. 1).
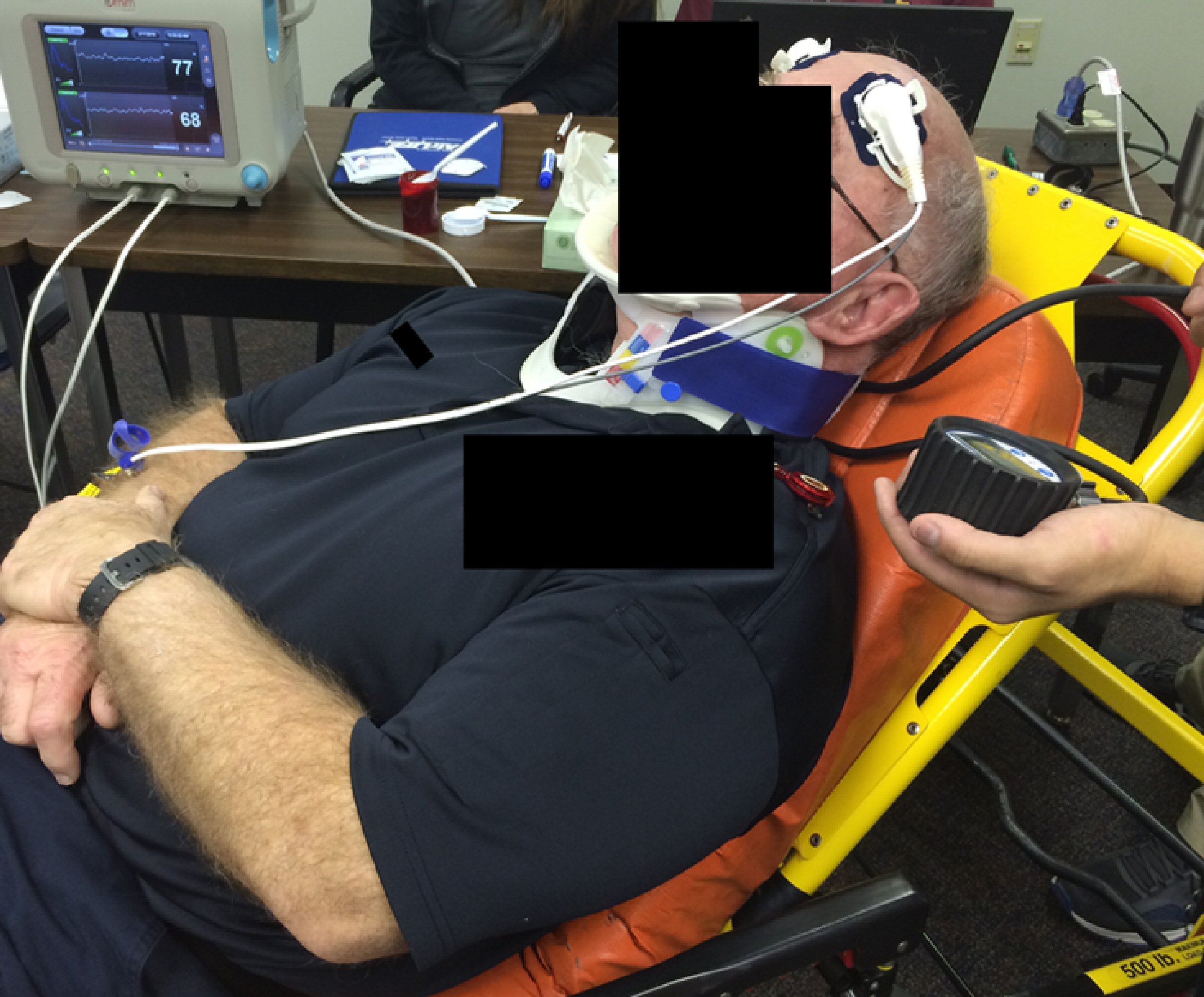 Click for large image | Figure 1. The study design with a sensor that is adhered to the skin. The monitor was applied to assess the changes of blood flow to the brain: before and after application of several c-collars, as the patient positions change from supine (with and without LSB), and as the head of the bed is elevated. c-collar: cervical collar; LSB: long spinal board. |
Participants
Healthy volunteers were recruited, underwent a full informed consent process, and were assessed to have no medically significant spinal abnormalities, obvious pregnancy, nor contraindications for monitor placement on areas with which the LSB, c-collar, or stretcher mattress may come into contact.
Exclusion criteria are: pregnancy, prisoners, hypertension, history of cardiovascular diseases, dermatologic problems, or any history of anxiety, nausea, dizziness, headache, neck pain, or currently taking anxiolytic or analgesic medications.
Procedure
After informed consent was obtained, each subject underwent an initial screening for inclusion and exclusion criteria. Subjects were fitted with the CBFI monitor and the monitor was allowed to establish a baseline index while the subject was relaxed in a comfortable position. Vital signs were monitored using standard non-invasive monitoring (blood pressure, pulse rate, oxygen saturation, and respiratory rate). Subjects were then provided a randomization package which indicated their order of positioning and whether the c-collar should be worn. Each subject was then tested in a random sequence of the following positions: standing with and without rigid c-collar, sitting upright with and without rigid c-collar, laying on EMS stretcher with head of bed elevated greater than 45, 30, and 10 or 15° with and without rigid c-collar, laying supine on EMS stretcher with and without rigid c-collar, and laying supine on EMS stretcher with LSB and rigid c-collar. Thus, there are a total of six positions with and six positions without c-collar. In all cases, the collar was fitted by a professional licensed paramedic as per manufacturer’s recommendations, and the head of the bed angle was determined using an electronic level. In each position, the subject was allowed to rest as to reestablish a stable perfusion index and, once stabilized, a 3-min perfusion recording period was documented.
Measurements
For each of the above described conditions, a 1-min stabilization period was followed by 3 min of cerebral perfusion monitoring. Measurements taken included mean, standard deviation (SD), 95% confidence interval (CI), and/or trending.
Data analysis
CBFI describes cerebral blood flow as an arbitrary number from 0 to 100. It is capable of setting a baseline flow value and presenting the change from this baseline. Descriptive statistics were utilized for the overall cerebral perfusion during each condition. For each position no collar was used as the comparator for that position, and the positioning was dichotomized as greater than or equal to 30°, or less than 30°. A t-test was utilized to identify any significance in change in cerebral blood flow, with significance determined by P ≤ 0.05.
Outcomes
The primary outcome was the change in CBFI under the conditions described above.
| Results | ▴Top |
Participants
Five subjects were recruited, and all completed the study in a single session. Each subject was tested in six positions both with and without the c-collar. Mean age of volunteers was 49 (SD: 15, 95% CI: 30.4 - 67.7) years and 60% male. Mean body mass index (BMI) was 29.4 (SD: 4.0, 95% CI: 24.4 - 34.3), and mean neck circumference was 40.1 cm (SD: 3.6, 95% CI: 35.7 - 44.5) (Table 1).
 Click to view | Table 1. Subject Demographics |
Main results
All subjects demonstrated a difference with respect to head of bed positioning dichotomized as either greater than or equal to 30° and less than 30° (Table 2). Mean of means for CBFI was 72.6 in the low angle cohort and 68.1 in the higher angle cohort. When comparing CBFI with and without the application of the c-collar, there were no significant differences in the CBFI for four of the five subjects. Mean of means for CBFI was 71.0 in those subjects with the c-collar on, and mean of means was 69.0 in the cohort without the c-collar. Subject four did demonstrate a significantly higher CBFI with the c-collar applied (mean: 72.8, SD: 6.7 vs. mean: 67.0, SD: 4.0; P = 0.0004) (Table 3). CBFI was stratified by head of bed angle and c-collar application (Supplementary Materials 1 and 2, www.neurores.org).
 Click to view | Table 2. Cerebral Blood Flow Index as a Function of Head of Bed Position, Dichotomized to Less Than Thirty Degrees, or Greater Than or Equal to Thirty Degrees |
 Click to view | Table 3. Comparing Cerebral Blood Flow Index With and Without C-Collar |
| Discussion | ▴Top |
From this pilot study, our aim was to understand the effects of c-collar application and head of bed elevation on cerebral hemodynamics. The application of collars and elevation to 30° has often become the standard of care in the acute management of trauma patients, especially in those patients who are unconscious or have evidence of neurologic deficits prior to complementary imaging tests [10]. In spite of this, little and inconsistent literature exists on the impact of this procedural choice on a patient’s cerebral blood flow. In this section, we discuss our findings in the context of the cerebrovascular physiology.
Relevant physiology
The brain is one of the most expensive organs of the human body in terms of energy expenditure. Weighing only 2%, the brain requires 20% of the body’s power consumption. This significant energy mismatch highlights the limited capacity of neurons to survive in anaerobic conditions and stresses the importance of maintained cerebral perfusion [11].
Cerebral blood flow is regulated by several factors, which include arterial blood pressure, intracranial pressure, blood viscosity, partial pressures of carbon dioxide and oxygen, vasoreactivity, and status of cerebral autoregulation [12-16]. The cerebral blood flow represents the blood volume per time per unit mass entering the cranial cavity, as supplied by the carotids anteriorly and the vertebral arteries posteriorly.
Following Ohm’s law for fluids, the cerebral blood flow, CBF, is the quotient of cerebral perfusion pressure (CPP), and cerebral vascular resistance (R), (CBF = CPP/R). The CPP represents the difference from the mean arterial pressure, MAP, which is the weighted average of the systolic and diastolic arterial pressures, and the intracranial pressure (ICP), (CPP = MAP - ICP). The cerebral vascular resistance is roughly estimated by Poiseuille’s law (R = 8 (viscosity) (length)/π (radius)4). Poiseuille’s law demonstrates the robustness of autoregulation, which maintains the cerebral blood flow with changes in CPP and prevents against the fatal consequences of hypoxia to the brain. Modest changes in cerebral vessel radius produces marked changes in the vascular resistance thereby maintaining flow [12]. This effect is the basis for some mechanisms including the myogenic, autonomic, and metabolic mechanisms that describe autoregulation [14-16]. Conservative estimates of the blood flow plateau phase of autoregulation range between 60 and 150 mm Hg in normotensive adults [14].
Impact of c-collars on cerebral blood flow
Restriction of cervical motion following trauma prevents secondary damage to a potentially unstable cervical spine; however, it is important to note that no method known to date offers complete restriction of movement. Nevertheless, c-collars are cumbersome and pose their own risks in patients. Airway compromise and elevations in ICP are commonly described in the literature. Specifically, rigid c-collars are theorized to increase jugular venous pressure from external compression on the superficial and compliant underlying veins of the neck. This impairs the venous outflow from the cranial cavity and can decrease cerebral blood flow by increasing ICP [17, 18]. C-collars are less likely to affect the CPP since the arterial system is less compliant and deeper within the neck. One study by Maissan et al demonstrated this increased ICP by showing that rigid c-collars have a greater tendency to increase ICP than semi-rigid collars. The group measured the optical nerve sheath diameter, as an indirect observation of ICP, in healthy volunteers [18].
In contrast, the results from our study demonstrated that the application of a rigid c-collar, as per manufacturer’s labeling, did not significantly affect the CBFI in all but one of our healthy volunteers. This may be largely due to the intact autoregulation mechanisms of our volunteers. Like that of Maissan’s study, it is possible that ICP was increased by application of the c-collar, but autoregulation allowed for maintenance of cerebral blood flow in our subjects. In a post-hoc analysis of our data, we stratified the head of bed elevation and application of the c-collar to account for any confounding factors causing deviation in subject 4 (Supplementary Materials 1 and 2, www.neurores.org). It is significant to note that subject 4 had a neck circumference that was at the upper range of the manufacturer’s size recommendations. We believe that a slightly larger c-collar would have minimized these effects, with moderate changes in the device’s restriction capability.
Impact of head of bed elevation on cerebral blood flow
The elevation of the head of bed is a simple and cheap intervention that is widely utilized in the management of acute and chronic trauma, especially in those with traumatic brain injury, intracranial hypertension, and spinal injury. Acutely, elevation of the head of bed is theorized to improve venous return to the heart from the cranial cavity and redistribute cerebrospinal fluid towards the spinal subarachnoid spaces [19]. This together would decrease ICP and hence increase CPP and cerebral blood flow in those patients without intact autoregulation. Additionally, during transport, changes in velocity of the transporting ambulance could theoretically have significant effects of the hemodynamics to the brain when the patient is laying completely flat in a head-forward orientation. This may allow blood inertia to increase the pressure to the cranial vault during braking [20]. Chronically, elevation of the head of bed also helps to reduce the risk of pressure ulcers, ventilator-associated pneumonias, and helps to provide patient support and comfort [21, 22].
Nonetheless, head of bed elevation poses a dilemma such that decreases in ICP are offset by increasing risks for cerebral ischemia in those with impaired or unstable autoregulation or MAP [19]. In our own study, we found head of bed elevation greater than or equal to 30° was significantly associated with decreased cerebral blood flow indices when compared against lesser angles for all healthy subjects. In patients with intact autoregulation it is possible that gradual changes in position do not affect the dynamic autoregulation. In one recent study by Garrett et al, there was an observed 13% reduction in cerebral blood volume when changing from supine to seated 90° posture in 18 young healthy volunteers [22]. Similarly, a recent analysis by Lam et al showed that dynamic autoregulation assessed over a 5-min time interval were not affected by gradual changes in head position, but did result in reproducible static changes in blood pressure and cerebral blood volume in 19 healthy volunteers [23]. From these analyses and our own, it seems that gradual position changes may not fully be accounted for by even intact autoregulatory mechanisms, which may especially have clinical relevance in those patients with impaired states.
The choice of 30° to dichotomize the analysis could have affected the statistics of our results, but it was not chosen arbitrarily. It is hypothesized that this position provides optimal support and stability for a patient in transport. Previous work has demonstrated that the stretcher mattress provides reduced motion as compared to transport on an LSB [20]. Thirty degrees also seems hypothetically important in reducing the effect of acceleration and breaking forces during the ambulance transport. Additionally, elevation at 30° or greater allows for the head to be above the heart in a way such that cerebral spinal fluid (CSF) is optimally redistributed from the cranial to the spinal subarachnoid spaces and thereby facilitate cerebral venous return [19].
Therefore, we must consider the effects of the head of bed angle in transport and acute management of trauma patients. While there are numerous cited benefits for head of bed elevation, these may be undermined by a lower cerebral perfusion pressure in critically ill patients. According to our own findings, and those supported by Rosner et al, supine or lower elevation angles are optimal if CPP is outweighed against acute ICP changes and transport accelerative forces, or chronic pressure ulcers, VAP, and comfort [17, 24]. It is critical to understand changes in head position in healthy individuals in order to optimize the management for those with severe head injury, post-operative neurosurgery, elevated ICP, trauma, and stroke [23].
Limitations
The main limitation of this project is that it was conducted using healthy volunteers with intact autoregulation in a static environment, where the brain quickly adjusts to changes in arterial blood pressure to maintain constant blood flow. This limits generalizability to the traumatic brain injured (TBI) patient, since we did not assess CBFI in the context of impaired autoregulation or adverse impact of the outcome on TBI patients. The autoregulation capability of the brain is different in different patient groups, especially with patients of various GCS scores [24].
Furthermore, the blood flow index being a unitless number makes it difficult to determine if a moderately higher number is improved. From the data obtained here, all that can be definitively stated is that the blood flow changes with positioning but does not substantively change with the use of the c-collar. Additionally, this was not a blinded study; all results were objective and recorded electronically, but the subject position and collar application were known to the subject and researchers although the subjects were blinded to the study hypothesis. Lastly, greater power is needed to evaluate the results from this pilot study. The deviation of subject 4 may have been accounted for by the constraints of a tighter c-collar or by statistical chance that may have been mitigated by larger sample size.
Conclusions
Our pilot study shows that c-collar application may not significantly affect cerebral blood flow. Elevation of the head of the bed is associated with significant reduction in CBFI. While this difference may not be clinically significant in healthy volunteers, this change in cerebral blood flow may have clinical significance in a TBI population. Continued research is needed to identify best practice for patient positioning during prehospital transport for protection of the cervical spine and optimization of cerebral blood flow. Further future prospective studies are warranted to evaluate these differences in a larger cohort.
| Supplementary Material | ▴Top |
Suppl 1. Cerebral Blood Flow Index Stratified by Cervical Collar Application.
Suppl 2. Cerebral Blood Flow Index Stratified by Head of Bed Angle.
Acknowledgments
None to declare.
Financial Disclosure
Research reported in this presentation was funded through a Department of Defense Grant Award (W81XWH-14-2-0149). The opinions stated are solely the responsibility of the authors and do not necessarily represent the views of the Department of Defense, Remote Trauma Outcome Research Network (RemTORN), or the Southwest Texas Regional Advisory Committee (STRAC).
Conflict of Interest
The authors report no conflict of interest.
Informed Consent
Informed consent was obtained.
Author Contributions
David Wampler served as the project PI, responsible for study design and regulatory issues, manuscript preparation. Brian Eastridge: RemTORN PI and contribution to study design, and manuscript drafting. Rena Summers: study design, subject recruitment and consent, and final manuscript revision. Preston Love: study design and data collection. Anand Dharia: data analysis, manuscript drafting, and final manuscript revision. Ali Seifi: project Co-PI, study design, data analysis, and manuscript drafting, and revision.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Hood N, Considine J. Spinal immobilisaton in pre-hospital and emergency care: A systematic review of the literature. Australas Emerg Nurs J. 2015;18(3):118-137.
doi pubmed - McDonald NE, Curran-Sills G, Thomas RE. Outcomes and characteristics of non-immobilised, spine-injured trauma patients: a systematic review of prehospital selective immobilisation protocols. Emerg Med J. 2016;33(10):732-740.
doi pubmed - PHTLS: prehospital trauma life support. Burlington, MA: Jones & Bartlett Learning; 2011.
- Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 Suppl):285-291.
doi pubmed - EMS spinal precautions and the use of the long backboard. Prehosp Emerg Care. 2013;17(3):392-393.
doi pubmed - Bruijns SR, Guly HR, Wallis LA. Effect of spinal immobilization on heart rate, blood pressure and respiratory rate. Prehosp Disaster Med. 2013;28(3):210-214.
doi pubmed - Horodyski M, DiPaola CP, Conrad BP, Rechtine GR, 2nd. Cervical collars are insufficient for immobilizing an unstable cervical spine injury. J Emerg Med. 2011;41(5):513-519.
doi pubmed - Sundstrom T, Asbjornsen H, Habiba S, Sunde GA, Wester K. Prehospital use of cervical collars in trauma patients: a critical review. J Neurotrauma. 2014;31(6):531-540.
doi pubmed - Schytz HW, Guo S, Jensen LT, Kamar M, Nini A, Gress DR, Ashina M. A new technology for detecting cerebral blood flow: a comparative study of ultrasound tagged NIRS and 133Xe-SPECT. Neurocrit Care. 2012;17(1):139-145.
doi pubmed - Nunez-Patino RA, Rubiano AM, Godoy DA. Impact of Cervical Collars on Intracranial Pressure Values in Traumatic Brain Injury: A Systematic Review and Meta-Analysis of Prospective Studies. Neurocrit Care. 2020;32(2):469-477.
doi pubmed - Fantini S, Sassaroli A, Tgavalekos KT, Kornbluth J. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics. 2016;3(3):031411.
doi pubmed - Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39(2):183-238.
doi pubmed - Kety SS, Schmidt CF. Measurement of cerebral blood flow and cerebral oxygen consumption in man. Fed Proc. 1946;5:264.
- Kelly DF, Martin NA, Kordestani R, Counelis G, Hovda DA, Bergsneider M, McBride DQ, et al. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg. 1997;86(4):633-641.
doi pubmed - Yang S-H, Liu R. Cerebral autoregulation. Primer on Cerebrovascular Diseases. 2017;57-60.
doi - Alois Zauner, Paul Muizelaar J. Brain metabolism and cerebral blood flow. Chapter 5.
- Rosner MJ, Coley IB. Cerebral perfusion pressure, intracranial pressure, and head elevation. J Neurosurg. 1986;65(5):636-641.
doi pubmed - Maissan IM, Ketelaars R, Vlottes B, Hoeks SE, den Hartog D, Stolker RJ. Increase in intracranial pressure by application of a rigid cervical collar: a pilot study in healthy volunteers. Eur J Emerg Med. 2018;25(6):e24-e28.
doi pubmed - Alarcon JD, Rubiano AM, Okonkwo DO, Urrutia G, Cosp XB. Elevation of the head during intensive care management in patients with severe traumatic brain injury. Cochrane Database of Systematic Reviews. 2012.
doi - Wampler DA, Pineda C, Polk J, Kidd E, Leboeuf D, Flores M, Shown M, et al. The long spine board does not reduce lateral motion during transport—a randomized healthy volunteer crossover trial. Am J Emerg Med. 2016;34(4):717-721.
doi pubmed - Olavarria VV, Lavados PM, Munoz-Venturelli P, Gonzalez F, Gaete J, Martins S, Arima H, et al. Flat-head positioning increases cerebral blood flow in anterior circulation acute ischemic stroke. A cluster randomized phase IIb trial. Int J Stroke. 2018;13(6):600-611.
doi pubmed - Garrett ZK, Pearson J, Subudhi AW. Postural effects on cerebral blood flow and autoregulation. Physiol Rep. 2017;5(4).
doi pubmed - Lam MY, Haunton VJ, Robinson TG, Panerai RB. Does gradual change in head positioning affect cerebrovascular physiology? Physiological Reports. 2018;6.
doi pubmed - Altun Ugras G, Yuksel S, Temiz Z, Eroglu S, Sirin K, Turan Y. Effects of different head-of-bed elevations and body positions on intracranial pressure and cerebral perfusion pressure in neurosurgical patients. J Neurosci Nurs. 2018;50(4):247-251.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
